Organopotassium compound on:
[Wikipedia]
[Google]
[Amazon]
Organosodium chemistry is the chemistry of
 Simple organosodium compounds such as the alkyl and aryl derivatives are generally insoluble polymers. Because of its large radius, Na prefers a higher coordination number than does lithium in organolithium compounds. Methyl sodium adopts a polymeric structure consisting of interconnected aCH3sub>4 clusters. When the organic substituents are bulky and especially in the presence of chelating ligands like TMEDA, the derivatives are more soluble. For example, aCH2SiMe3MEDA is soluble in hexane. Crystals have been shown to consist of chains of alternating Na(TMEDA)+ and CH2SiMe groups with Na–C distances ranging from 2.523(9) to 2.643(9) Å.William Clegg, Ben Conway, Alan R. Kennedy, Jan Klett, Robert E. Mulvey, Luca Russo "Synthesis and Structures of Trimethylsilyl)methylodium and -potassium with Bi- and Tridentate N-Donor Ligands" Eur. J. Inorg. Chem. 2011, pp. 721–726.
Simple organosodium compounds such as the alkyl and aryl derivatives are generally insoluble polymers. Because of its large radius, Na prefers a higher coordination number than does lithium in organolithium compounds. Methyl sodium adopts a polymeric structure consisting of interconnected aCH3sub>4 clusters. When the organic substituents are bulky and especially in the presence of chelating ligands like TMEDA, the derivatives are more soluble. For example, aCH2SiMe3MEDA is soluble in hexane. Crystals have been shown to consist of chains of alternating Na(TMEDA)+ and CH2SiMe groups with Na–C distances ranging from 2.523(9) to 2.643(9) Å.William Clegg, Ben Conway, Alan R. Kennedy, Jan Klett, Robert E. Mulvey, Luca Russo "Synthesis and Structures of Trimethylsilyl)methylodium and -potassium with Bi- and Tridentate N-Donor Ligands" Eur. J. Inorg. Chem. 2011, pp. 721–726.

organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
to sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
The principal organosodium compound of commercial importance is sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are p ...
. Sodium tetraphenylborate
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are o ...
can also be classified as an organosodium compound since in the solid state sodium is bound to the aryl groups.
Organometal bonds in group 1 are characterised by high polarity with corresponding high nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
on carbon. This polarity results from the disparate electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of carbon (2.55) and that of lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
0.98, sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
0.93 potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
0.82 rubidium 0.82 caesium 0.79). The carbanionic nature of organosodium compounds can be minimized by resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
, for example, Ph3CNa. One consequence of the highly polarized Na-C bond is that simple organosodium compounds often exist as polymers that are poorly soluble in solvents.
Synthesis
Transmetallation routes
In the original work the alkylsodium compound was accessed from the dialkylmercury compound by transmetallation. For example,diethylmercury
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous.
This chemical c ...
in the Schorigin reaction or Shorygin reaction:Dietmar Seyferth "Alkyl and Aryl Derivatives of the Alkali Metals: Strong Bases and Reactive Nucleophiles. 2. Wilhelm Schlenk's Organoalkali-Metal Chemistry. The Metal Displacement and the Transmetalation Reactions. Metalation of Weakly Acidic Hydrocarbons. Superbases" Organometallics 2009, volume 28, pp 2–33.
:(C2H5)2Hg + 2 Na → 2 C2H5Na + Hg
The high solubility of lithium alkoxides in hexane is the basis of a useful synthetic route:
:LiCH2SiMe3 + NaO–t–Bu → LiOt–Bu + NaCH2SiMe3
Deprotonation routes
For some acidic organic compounds, the corresponding organosodium compounds arise by deprotonation.Sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are p ...
is thus prepared by treating sodium metal and cyclopentadiene:
:2 Na+ 2 C5H6 → 2 Na+ C5H5− + H2
Sodium acetylides form similarly. Often strong sodium bases are employed in place of the metal. Sodium methylsulfinylmethylide
Sodium methylsulfinylmethylide (also called NaDMSO or dimsyl sodium) is the sodium salt of the conjugate base of dimethyl sulfoxide. This unusual salt has some uses in organic chemistry as a base and nucleophile.
Since the first publication in 19 ...
is prepared by treating DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds ...
with sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
:
:CH3SOCH3 + NaH → CH3SOCHNa+ + H2
Metal-halogen exchange
Trityl sodium can be prepared by sodium-halogen exchange: :Ph3CCl + 2 Na → Ph3C− Na+ + NaClElectron transfer
Sodium also reacts withpolycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. ...
s via one-electron reduction
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
. With solutions of naphthalene, it forms the deeply coloured radical sodium naphthalene
Sodium naphthalene is an organic salt with the chemical formula Na+. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When isolated, it ...
, which is used as a soluble reducing agent:
:C10H8 + Na → Na+ 10H8sup>−•
Structural studies show however that sodium naphthalene has no Na-C bond, the sodium is invariably coordinated by ether or amine ligands. The related anthracene as well as lithium derivatives are well known.
Structures
 Simple organosodium compounds such as the alkyl and aryl derivatives are generally insoluble polymers. Because of its large radius, Na prefers a higher coordination number than does lithium in organolithium compounds. Methyl sodium adopts a polymeric structure consisting of interconnected aCH3sub>4 clusters. When the organic substituents are bulky and especially in the presence of chelating ligands like TMEDA, the derivatives are more soluble. For example, aCH2SiMe3MEDA is soluble in hexane. Crystals have been shown to consist of chains of alternating Na(TMEDA)+ and CH2SiMe groups with Na–C distances ranging from 2.523(9) to 2.643(9) Å.William Clegg, Ben Conway, Alan R. Kennedy, Jan Klett, Robert E. Mulvey, Luca Russo "Synthesis and Structures of Trimethylsilyl)methylodium and -potassium with Bi- and Tridentate N-Donor Ligands" Eur. J. Inorg. Chem. 2011, pp. 721–726.
Simple organosodium compounds such as the alkyl and aryl derivatives are generally insoluble polymers. Because of its large radius, Na prefers a higher coordination number than does lithium in organolithium compounds. Methyl sodium adopts a polymeric structure consisting of interconnected aCH3sub>4 clusters. When the organic substituents are bulky and especially in the presence of chelating ligands like TMEDA, the derivatives are more soluble. For example, aCH2SiMe3MEDA is soluble in hexane. Crystals have been shown to consist of chains of alternating Na(TMEDA)+ and CH2SiMe groups with Na–C distances ranging from 2.523(9) to 2.643(9) Å.William Clegg, Ben Conway, Alan R. Kennedy, Jan Klett, Robert E. Mulvey, Luca Russo "Synthesis and Structures of Trimethylsilyl)methylodium and -potassium with Bi- and Tridentate N-Donor Ligands" Eur. J. Inorg. Chem. 2011, pp. 721–726.
Reactions
Organosodium compounds are traditionally used as strong bases, although this application has been supplanted by other reagents such assodium bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula . This species, usually called NaHMDS (sodium hexamethyldisilazide), is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are ...
.
The higher alkali metals are known to metalate even some unactivated hydrocarbons and are known to self-metalate:
: 2 NaC2H5 → C2H4Na2 + C2H6
In the Wanklyn reaction (1858) organosodium compounds react with carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
to give carboxylates:
:C2H5Na + CO2 → C2H5CO2Na
Grignard reagents undergo a similar reaction.
Some organosodium compounds degrade by beta-elimination:
:NaC2H5 → NaH + C2H4
Industrial applications
Although organosodium chemistry has been described to be of "little industrial importance", it once was central to the production oftetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula Pb( C2H5)4. It is a fuel additive, first being mixed with gasoline beginning in the 1920s as a patented octane rating booster that ...
. A similar Wurtz coupling
In organic chemistry, the Wurtz reaction, named after Charles Adolphe Wurtz, is a coupling reaction whereby two alkyl halides are treated with sodium metal to form a higher alkane.
: 2 R−X + 2 Na → R−R + 2 NaX
The reaction is of little v ...
-like reaction is the basis of the industrial route to triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
:
:3 PhCl + PCl3 + 6 Na → PPh3 + 6 NaCl
The polymerization of butadiene and styrene is catalyzed by sodium metal.
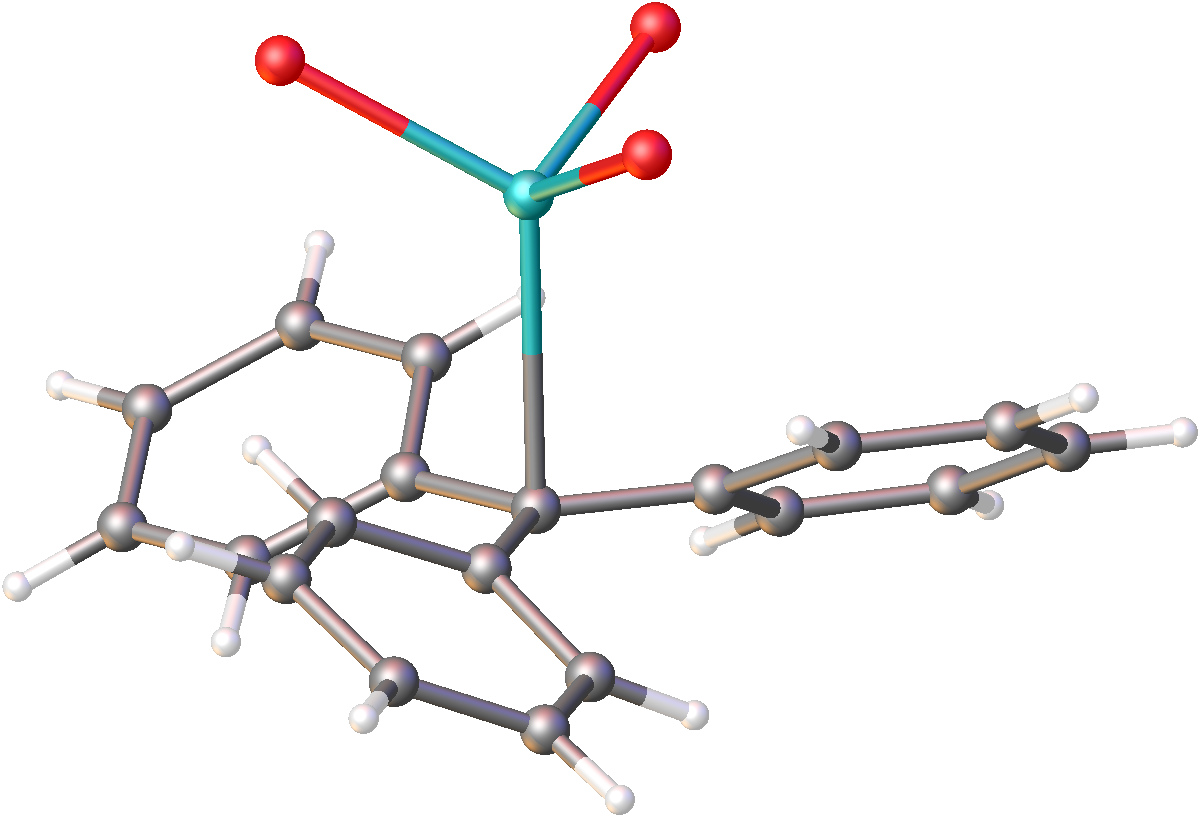
Organic derivatives of the heavier alkali metals
Organopotassium, organorubidium, and organocaesium are less commonly encountered than organosodium compounds and of limited utility. These compounds can be prepared by treatment of alkyl lithium compounds with the potassium, rubidium, and caesium alkoxides. Alternatively they arise from the organomercury compound, although this method is dated. The solid methyl derivatives adopt polymeric structures. Reminiscent of thenickel arsenide
Nickeline or niccolite is a mineral consisting primarily of nickel arsenide (NiAs). The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly.
Small quantities of ...
structure, MCH3 (M = K, Rb, Cs) has six alkali metal centers bound to each methyl group. The methyl groups are pyramidal, as expected.E. Weiss, "Structures of Organo Alkali Metal Complexes and Related Compounds" Angewandte Chemie International Edition in English, 1993, volume 32, pages 1501–1523.
A notable reagent that is based on a heavier alkali metal alkyl is Schlosser's base Schlosser's base (or Lochmann-Schlosser base) describes various superbasic mixtures of an alkyllithium compound and a potassium alkoxide. The reagent is named after Manfred Schlosser, although he uses the term ''LICKOR superbase'' (LIC denoting the ...
, a mixture of ''n''-butyllithium and potassium ''tert''-butoxide. This reagent reacts with toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) a ...
to form the red-orange compound benzyl potassium
Benzylpotassium is an organopotassium compound with the formula C6H5CH2K. It is an orange powder. Like organo-alkali metal reagents in general, benzyl potassium is highly reactive, so much so that it reacts with most solvents. It is highly air s ...
(KCH2C6H5).
Evidence for the formation of heavy alkali metal-organic intermediates is provided by the equilibration of ''cis''-but-2-ene and ''trans''-but-2-ene catalysed by alkali metals. The isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
is fast with lithium and sodium, but slow with the higher alkali metals. The higher alkali metals also favor the sterically congested conformation. Several crystal structures of organopotassium compounds have been reported, establishing that they, like the sodium compounds, are polymeric.
See also
*Alkynation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an α-alkynyl alcohol ().
When the acetylide is formed from acetylene (), the reaction gives an α- ethynyl alcoho ...
References
{{ChemicalBondsToCarbon