Organopalladium on:
[Wikipedia]
[Google]
[Amazon]
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic

 Palladium(II) acetate and related compounds are common reagents because the carboxylates are good leaving groups with basic properties. For example palladium trifluoroacetate has been demonstrated to be effective in aromatic decarboxylation:
Palladium(II) acetate and related compounds are common reagents because the carboxylates are good leaving groups with basic properties. For example palladium trifluoroacetate has been demonstrated to be effective in aromatic decarboxylation:
 Allylpalladium intermediates also feature in the Trost asymmetric allylic alkylation and the Carroll rearrangement and an oxo variation in the Saegusa oxidation.
Allylpalladium intermediates also feature in the Trost asymmetric allylic alkylation and the Carroll rearrangement and an oxo variation in the Saegusa oxidation.
 The hydride shift was envisaged as taking place through a Pd(IV)
The hydride shift was envisaged as taking place through a Pd(IV)  In related work the intermediate associated with the hydride shift remains Pd(II):
:
In related work the intermediate associated with the hydride shift remains Pd(II):
: and in other work (a novel synthesis of
and in other work (a novel synthesis of  and in certain intramolecular couplings synthetic value was demonstrated regardless of oxidation state:''Pd-Catalyzed Alkyl to Aryl Migration and Cyclization: An Efficient Synthesis of Fused Polycycles via Multiple C-H Activation'' Qinhua Huang, Alessia Fazio, Guangxiu Dai, Marino A. Campo, and Richard C. Larock '' J. Am. Chem. Soc.'' 2004, 126, 7460-7461
:
and in certain intramolecular couplings synthetic value was demonstrated regardless of oxidation state:''Pd-Catalyzed Alkyl to Aryl Migration and Cyclization: An Efficient Synthesis of Fused Polycycles via Multiple C-H Activation'' Qinhua Huang, Alessia Fazio, Guangxiu Dai, Marino A. Campo, and Richard C. Larock '' J. Am. Chem. Soc.'' 2004, 126, 7460-7461
:
palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s and alkynes with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
s, as demonstrated in tandem reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
s.
Organopalladium chemistry timeline
* 1873 - A. N. Zaitsev reports reduction ofbenzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylket ...
over palladium with hydrogen.
* 1894 - Phillips reports that palladium(II) chloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value ...
reduces to palladium metal by contact with ethylene.
* 1907 - Autoclave
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform sterilizati ...
technology introduced by Vladimir Ipatieff
Vladimir Nikolayevich Ipatieff (also Ipatyev; russian: Владимир Николаевич Ипатьев); (November 21, 1867 (November 9 OS) – November 29, 1952) was a Russian and American chemist. His most important contributions are in the ...
makes it possible to carry out high pressure hydrogenation.
* 1956 - In the Wacker process ethylene and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
react to acetaldehyde with catalyst PdCl2/CuCl2
* 1957 - Tetrakis(triphenylphosphine)palladium(0) is reported by Malatesta and Angoletta.
* 1972 - The Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a sub ...
is a coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
of a halogenide with an olefin. Pd(0) intermediates are implicated.
* 1973 - The Trost asymmetric allylic alkylation is a nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
.
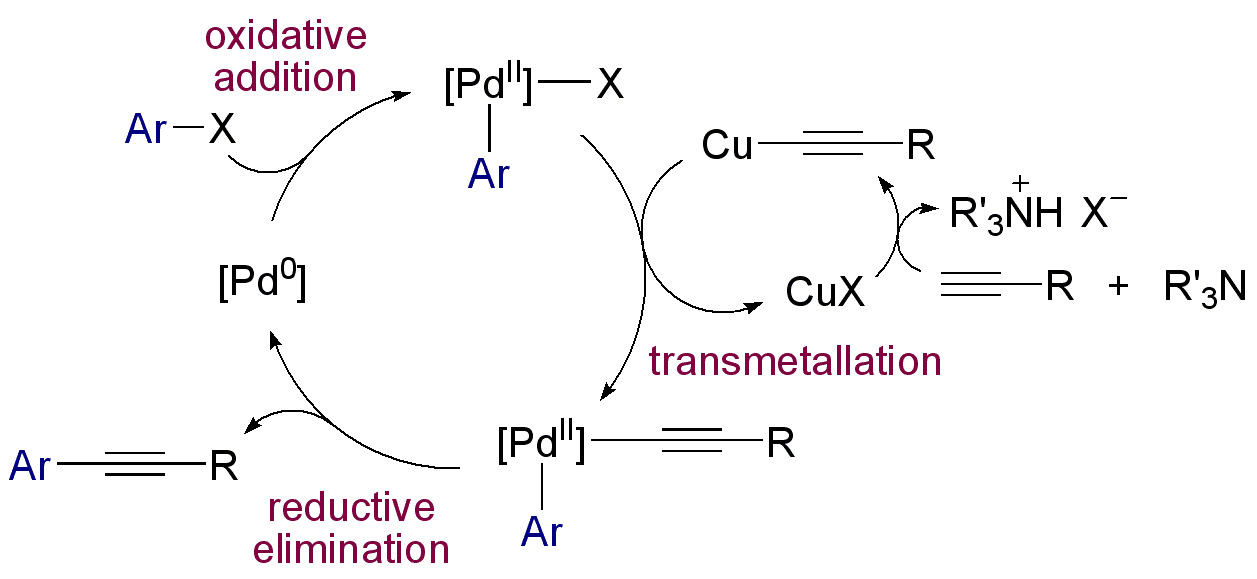
* 1975 - The Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or v ...
is a coupling reaction of terminal alkynes with aryl or vinyl halides.
* 1994 - The Pd-catalyzed Buchwald-Hartwig amination for C-N bond-forming reactions.

Palladium(II)
Alkene complexes
Unlike Ni(II), but similar to Pt(II), Pd(II) halides form a variety of alkene complexes. The premier example isdichloro(1,5‐cyclooctadiene)palladium
Dichloro(1,5-cyclooctadiene)palladium is the organopalladium compound with the formula PdCl2(C8H12) where C8H12 is cycloocta-1,5-diene (cod) or abbreviated PdCl2(cod). It is a yellow solid that is soluble in chloroform. According to X-ray crystal ...
. In this complex, the diene is easily displaced, which makes it a favored precursor to catalysts. In the industrially important Wacker process, ethylene is converted to acetaldehyde via nucleophilic attack of hydroxide on a Pd(II)-ethylene intermediate followed by formation of a vinyl alcohol complex. Fullerene ligands also bind with palladium(II).
 Palladium(II) acetate and related compounds are common reagents because the carboxylates are good leaving groups with basic properties. For example palladium trifluoroacetate has been demonstrated to be effective in aromatic decarboxylation:
Palladium(II) acetate and related compounds are common reagents because the carboxylates are good leaving groups with basic properties. For example palladium trifluoroacetate has been demonstrated to be effective in aromatic decarboxylation:
Allyl complexes
The iconic complex in this series isallylpalladium chloride dimer
Allylpalladium(II) chloride dimer (APC) is a chemical compound with the chemical formula, formula hapticity, η3-C3H5)PdCl. This yellow air-stable compound is an important catalyst used in organic synthesis.Tatsuno, Y.; Yoshida, T.; Otsuka, S. ...
(APC). Allyl compounds with suitable leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
s react with palladium(II) salts to pi-allyl complexes having hapticity
In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a l ...
3. These intermediates too react with nucleophiles for example carbanions derived from malonate esters or with amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s in allylic amination as depicted below
:Palladium-carbon sigma-bonded complexes
Various organic groups can bound to palladium and form stable sigma-bonded complexes. The stability of the bonds in terms of bond dissociation energy follows the trend: Pd-Alkynyl > Pd-Vinyl ≈ Pd-Aryl > Pd-Alkyl and the metal-carbon bond length changes in the opposite direction: Pd-Alkynyl < Pd-Vinyl ≈ Pd-Aryl < Pd-Alkyl.Palladium(0) compounds
Zerovalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
Pd(0) compounds include tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0) or d2(dba)3is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Becau ...
and tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound d(P(C6H5)3)4 often abbreviated Pd( PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon de ...
. These complexes react with halocarbon R-X in oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxid ...
to R-Pd-X intermediates with covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
Pd-C bonds. This chemistry forms the basis of a large class of organic reactions called coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
s (see palladium-catalyzed coupling reactions
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
). An example is the Sonogashira reaction:
:
Organopalladium(IV)
The first organopalladium(IV) compound was described in 1986. This complex is Me3Pd(IV)(I)bpy (bpy = bidentate2,2'-bipyridine
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline ...
ligand) It was synthesized by oxidative addition of methyl iodide to Me2Pd(II)bpy.
Palladium compounds owe their reactivity to the ease of interconversion between Pd(0) and palladium(II) intermediates. There is no conclusive evidence however for the involvement of Pd(II) to Pd(IV) conversions in palladium mediated organometallic reactions. One reaction invoking such mechanism was described in 2000 and concerned a Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a sub ...
. This reaction was accompanied by a 1,5-hydrogen shift in the presence of amines:
:metallacycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates ...
:
:indole
Indole is an aromatic heterocyclic organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other c ...
s with two Pd migrations) equilibria are postulated between different palladacycles:
:References
{{ChemicalBondsToCarbon