Organocobalt chemistry on:
[Wikipedia]
[Google]
[Amazon]
Organocobalt chemistry is the chemistry of
 Most fundamental are the cobalt complexes with only alkyl ligands. Examples include Co(4-norbornyl)4 and its cation.
Alkylcobalt is represented by vitamin B12 and related enzymes. In
Most fundamental are the cobalt complexes with only alkyl ligands. Examples include Co(4-norbornyl)4 and its cation.
Alkylcobalt is represented by vitamin B12 and related enzymes. In
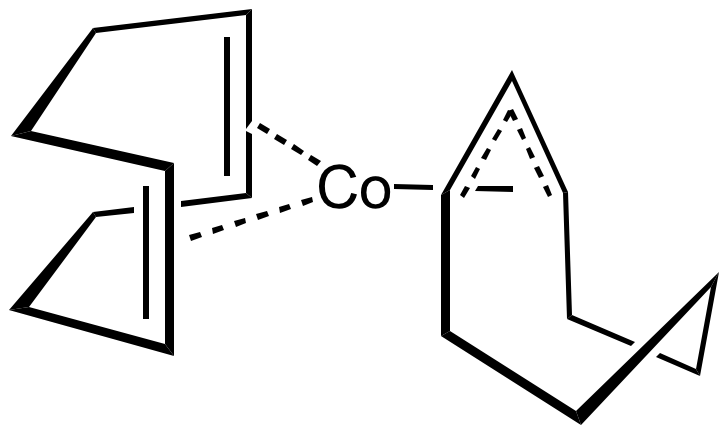 Organocobalt compounds are known with alkene, allyl, diene, and Cp ligands. A famous
Organocobalt compounds are known with alkene, allyl, diene, and Cp ligands. A famous
organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
to cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent me ...
.
Alkyl complexes
methylcobalamin
Methylcobalamin (mecobalamin, MeCbl, or MeB) is a cobalamin, a form of vitamin B. It differs from cyanocobalamin in that the cyano group at the cobalt is replaced with a methyl group. Methylcobalamin features an octahedral cobalt(III) centre and ...
the ligand is a methyl group, which is electrophilic. in vitamin B12, the alkyl ligand is an adenosyl group. Related to vitamin B12 are cobalt porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
s, dimethylglyoximates, and related complexes of Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
ligands. These synthetic compounds also form alkyl derivatives that undergo diverse reactions reminiscent of the biological processes. The weak cobalt(III)-carbon bond in vitamin B12 analogues can be exploited in a type of Cobalt mediated radical polymerization of acrylic and vinyl esters (e.g. vinyl acetate
Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate and ethene-vinyl acetate copolymers, important industrial polymers.
Production
The worldwide production capacity of v ...
), acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
and acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecula ...
.
Carbonyl complexes
Dicobalt octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent me ...
is produced by the carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbony ...
of cobalt salts. It and its phosphine derivatives are among the most widely used organocobalt compounds. Heating Co2(CO)8 gives Co4(CO)12. Very elaborate cobalt-carbonyl clusters have been prepared starting from these complexes. Heating cobalt carbonyl with bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluorofor ...
gives methylidynetricobaltnonacarbonyl
Methylidynetricobaltnonacarbonyl is the organocobalt compound with the formula HCCo3(CO)9. It is a metal carbonyl cluster that contains the methylidyne ligand. The compound has C3v point group symmetry. It is a purple, air-stable solid that is ...
. Dicobalt octacarbonyl also reacts with alkynes to give "tetrahedranes" of the formula Co2(CO)6(C2R2). Because the cobalt carbonyl centers can be removed later, it functions as a protective group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
for the alkyne. In the Nicholas reaction The Nicholas reaction is an organic reaction where a dicobalt octacarbonyl-stabilized propargylic cation is reacted with a nucleophile. Oxidative demetallation gives the desired alkylated alkyne. It is named after Kenneth M. Nicholas.
S ...
an alkyne group is also protected and at the same time the alpha-carbon position is activated for nucleophilic substitution.
Cp, allyl, and alkene compounds
Sandwich compounds
 Organocobalt compounds are known with alkene, allyl, diene, and Cp ligands. A famous
Organocobalt compounds are known with alkene, allyl, diene, and Cp ligands. A famous sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic deriv ...
is cobaltocene
Cobaltocene, known also as bis(cyclopentadienyl)cobalt(II) or even "bis Cp cobalt", is an organocobalt compound with the formula Co(C5H5)2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovere ...
, a rare example of low-spin Co(II) complex. This 19-electron metallocene is used as a reducing agent and as a source of CpCo. Other sandwich compounds are CoCp(C6Me6) and Co(C6Me6)2, with 20 electrons and 21 electrons, respectively. Reduction of anhydrous cobalt(II) chloride with sodium in the presence of cyclooctadiene gives Co(cyclooctadiene)(cyclooctenyl), a synthetically versatile reagent.
CpCo(CO)2 and derivatives
The half-sandwich compounds of the type CpCoL2 have been well investigated (L = CO, alkene). The complexes CpCo(C2H4)2 and CpCo(cod) catalyzealkyne trimerisation
In organic chemistry, an alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applic ...
, which has been applied to the synthesis of a variety of complex structures.
Applications
Dicobalt octacarbonyl is used commercially forhydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
of alkenes. A key intermediate is cobalt tetracarbonyl hydride (HCo(CO)4). Processes involving cobalt are practiced commercially mainly for the production of C7-C14 alcohols used for the production of surfactants.Boy Cornils, Wolfgang A. Herrmann, Chi-Huey Wong, Horst Werner Zanthoff: ''Catalysis from A to Z: A Concise Encyclopedia'', 2408 Seiten, Verlag Wiley-VCH Verlag GmbH & Co. KGaA, (2012), . Many hydroformylations have switched from cobalt-based processes to rhodium-based processes, despite the great expense of that metal. Replacing H2 by water or an alcohol, the reaction product is a carboxylic acid or an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
. An example of this reaction type is the conversion of butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vi ...
to adipic acid
Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annuall ...
. Cobalt catalysts (together with iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
) are relevant in the Fischer–Tropsch process
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatu ...
in which it is assumed that organocobalt intermediates form.
Cobalt complexes have been applies to the synthesis of pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ...
derivatives starting from alkynes and nitriles.
Aspirational applications
Although really only dicobalt octacarbonyl has achieved commercial success, many reports have appeared promising applications. Often these ventures are motivated by the use of "earth abundant" catalysts.References
{{ChemicalBondsToCarbon