Metal ions in aqueous solution on:
[Wikipedia]
[Google]
[Amazon]
A metal ion in aqueous solution or aqua ion is a
* No experimental information regarding aqua ion structures
Most  In aqueous solution the water molecules directly attached to the metal ion are said to belong to the
In aqueous solution the water molecules directly attached to the metal ion are said to belong to the
 The ions of these metals in the +2 and +3
The ions of these metals in the +2 and +3

 :
Other values include Zn2+ -2044.3, Cd2+ -1805.8 and Ag+ -475.3 kJ mol−1.
There is an excellent linear correlation between hydration enthalpy and the ratio of charge squared, z2, to M-O distance, reff.
:
Values for transition metals are affected by
:
Other values include Zn2+ -2044.3, Cd2+ -1805.8 and Ag+ -475.3 kJ mol−1.
There is an excellent linear correlation between hydration enthalpy and the ratio of charge squared, z2, to M-O distance, reff.
:
Values for transition metals are affected by
Al3+, Y3+, La3+, , , - , Li+, Na+, K+
Be2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+
Sc3+, Ti3+, V3+, Cr3+, Fe3+, Rh3+, Ga3+, In3+
Ce4+, Th4+, Pa4+, U4+, Np4+, Pu4+, , , , - , Ag+, Tl+
Pb2+
Ti3+, Bi3+, , , , - , Sn2+, Hg2+, Sn2+, Pd2+ , , ca. 12 The cations most resistant to hydrolysis for their size and charge are hard pre-transition metal ions or lanthanide ions. The slightly less resistant group includes the transition metal ions. The third group contains mostly soft ions ion of post-transition metals. The ions which show the strongest tendency to hydrolyze for their charge and size are Pd2+, Sn2+ and Hg2+. The standard enthalpy change for the first hydrolysis step is generally not very different from that of the dissociation of pure water. Consequently, the standard enthalpy change for the substitution reaction : (H2O)nsup>z+ +OH− : (H2O)n-1(OH)sup>(z-1)+ + H2O is close to zero. This is typical of reactions between a hard cation and a hard anion, such as the hydroxide ion. It means that the standard entropy charge is the major contributor to the standard free energy change and hence the equilibrium constant. : The change in ionic charge is responsible for the effect as the aqua ion has a greater ordering effect on the solution than the less highly charged hydroxo complex.
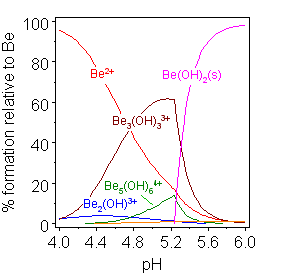

 The hydrolysis of beryllium shows many of the characteristics typical of multiple hydrolysis reactions. The concentrations of various species, including polynuclear species with bridging hydroxide ions, change as a function of pH up to the precipitation of an insoluble hydroxide. Beryllium hydrolysis is unusual in that the concentration of e(H2O)3(OH)sup>+ is too low to be measured. Instead a trimer ([Be3(H2O)6(OH3))3+ is formed, whose structure has been confirmed in solid salts. The formation of polynuclear species is driven by the reduction in charge density within the molecule as a whole. The local environment of the beryllium ions approximates to [Be(H2O)2(OH)2]+. The reduction in effective charge releases free energy in the form of a decrease of the entropy of ordering at the charge centers.
:{, class="wikitable"
, +Some polynuclear hydrolysis products
! Species formula!! cations!! ! scope="col" width="300" , structure
, -
, M2(OH)+, , Be2+, Mn2+, Co2+, Ni2+
The hydrolysis of beryllium shows many of the characteristics typical of multiple hydrolysis reactions. The concentrations of various species, including polynuclear species with bridging hydroxide ions, change as a function of pH up to the precipitation of an insoluble hydroxide. Beryllium hydrolysis is unusual in that the concentration of e(H2O)3(OH)sup>+ is too low to be measured. Instead a trimer ([Be3(H2O)6(OH3))3+ is formed, whose structure has been confirmed in solid salts. The formation of polynuclear species is driven by the reduction in charge density within the molecule as a whole. The local environment of the beryllium ions approximates to [Be(H2O)2(OH)2]+. The reduction in effective charge releases free energy in the form of a decrease of the entropy of ordering at the charge centers.
:{, class="wikitable"
, +Some polynuclear hydrolysis products
! Species formula!! cations!! ! scope="col" width="300" , structure
, -
, M2(OH)+, , Be2+, Mn2+, Co2+, Ni2+
Zn2+, Cd2+, Hg2+, Pb2+ , , single hydroxide bridge between two cations , - , M2(OH) , , Cu2+, Sn2+
Al3+, Sc3+, Ln3+, Ti3+, Cr3+
Th4+
VO2+, , , , , double hydroxide bridge between two cations , - , , , Be2+, Hg2+ , , six-membered ring with alternate Mn+ and OH− groups , - , (OH) , , Sn2+, Pb2+
Al3+, Cr3+, Fe3+, In3+ , , Cube with alternate vertices of Mn+ and OH− groups, one vertex missing , - , , , Mg2+, Co2+, Ni2+, Cd2+, Pb2+ , , Cube with alternate vertices of Mn+ and OH− groups , - , , , Zr4+, Th4+ , , Square of Mn+ ions with double hydroxide bridges on each side of the square The hydrolysis product of
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
, dissolved in water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, of chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
(H2O)nsup>z+. The solvation number, ''n'', determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for most elements in periods 3 and 4 of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
and actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
aqua ions have higher solvation numbers (often 8 to 9), with the highest knowm being 11 for Ac3+. The strength of the bonds between the metal ion and water molecules in the primary solvation shell
A solvation shell or solvation sheath is the solvent interface of any chemical compound or biomolecule that constitutes the solute. When the solvent is water it is called a hydration shell or hydration sphere. The number of solvent molecules sur ...
increases with the electrical charge, ''z'', on the metal ion and decreases as its ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the catio ...
, ''r'', increases. Aqua ions are subject to hydrolysis. The logarithm of the first hydrolysis constant is proportional to ''z''2/''r'' for most aqua ions.
The aqua ion is associated, through hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
ing with other water molecules in a secondary solvation shell. Water molecules in the first hydration shell exchange with molecules in the second solvation shell and molecules in the bulk liquid. The residence time of a molecule in the first shell varies among the chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s from about 100 picosecond
A picosecond (abbreviated as ps) is a unit of time in the International System of Units (SI) equal to 10−12 or (one trillionth) of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000&nbs ...
s to more than 200 years. Aqua ions are prominent in electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an out ...
.
Introduction to metal aqua ions
: :chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s are metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
lic. Compounds of the metallic elements usually form simple aqua ions with the formula (H2O)nsup>z+ in low oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s. With the higher oxidation states the simple aqua ions dissociate
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid ...
losing hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle ...
s to yield complexes that contain both water molecules and hydroxide or oxide ions, such as the vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pass ...
(IV) species O(H2O)5sup>2+. In the highest oxidation states only oxyanion An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determine ...
s, such as the permanganate
A permanganate () is a chemical compound containing the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a ...
(VII) ion, , are known. Some elements, such as tin and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient ti ...
, are clearly metals, but form only covalent compounds in the highest oxidation states. The transactinide
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s have been greyed out due to a lack of experimental data. For some highly radioactive elements, experimental chemistry has been done, and periodicity suggests that aqua ions were formed; but no experimental information is available regarding the structure of those putative aqua ions.
first coordination sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ...
, also known as the first, or primary, solvation shell. The bond between a water molecule and the metal ion is a dative covalent bond, with the oxygen atom donating both electrons to the bond. Each coordinated water molecule may be attached by hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
s to other water molecules. The latter are said to reside in the second coordination sphere. The second coordination sphere is not a well defined entity for ions with charge 1 or 2. In dilute solutions it merges into the water structure in which there is an irregular network of hydrogen bonds between water molecules. With tripositive ions the high charge on the cation polarizes the water molecules in the first solvation shell to such an extent that they form strong enough hydrogen bonds with molecules in the second shell to form a more stable entity.
The strength of the metal-oxygen bond can be estimated in various ways. The hydration enthalpy, though based indirectly on experimental measurements, is the most reliable measure. The scale of values is based on an arbitrarily chosen zero, but this does not affect differences between the values for two metals. Other measures include the M–O vibration frequency and the M–O bond length. The strength of the M-O bond tends to increase with the charge and decrease as the size of the metal ion increases. In fact there is a very good linear correlation between hydration enthalpy and the ratio of charge squared to ionic radius, z2/r. For ions in solution Shannon's "effective ionic radius" is the measure most often used.
Water molecules in the first and second solvation shells can exchange places. The rate of exchange varies enormously, depending on the metal and its oxidation state. Metal aqua ions are always accompanied in solution by solvated anions, but much less is known about anion solvation than about cation solvation.
Understanding of the nature of aqua ions is helped by having information on the nature of solvated cations in mixed solvents and non-aqueous solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s, such as liquid ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
, methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
, dimethyl formamide and dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds a ...
to mention a few.
Occurrence in nature
Aqua ions are present in most natural waters.Stumm&Morgan Na+, K+, Mg2+ and Ca2+ are major constituents ofseawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
.
:
Many other aqua ions are present in seawater in concentrations ranging from ppm to ppt. The concentrations of sodium, potassium, magnesium and calcium in blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the cir ...
are similar to those of seawater. Blood also has lower concentrations of essential element
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight ( oxygen, hydrogen, ca ...
s such as iron and zinc. Sports drink
Sports drinks, also known as electrolyte drinks, are functional beverages whose stated purpose is to help athletes replace water, electrolytes, and energy before, during and especially after training or competition. There are many perceived bene ...
is designed to be isotonic and also contains the minerals which are lost in perspiration
Perspiration, also known as sweating, is the production of fluids secreted by the sweat glands in the skin of mammals.
Two types of sweat glands can be found in humans: eccrine glands and apocrine glands. The eccrine sweat glands are distr ...
.
Magnesium and calcium ions are common constituents of domestic water and are responsible for permanent and temporary hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
, respectively. They are often found in mineral water
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. Mineral water may usually be still or sparkling (carbonated/effervescent) according to the presence or absence of added gases.
T ...
.
Experimental methods
Information obtained on the nature of ions in solution varies with the nature of the experimental method used. Some methods reveal properties of the cation directly, others reveal properties that depend on both cation and anion. Some methods supply information of a static nature, a kind of snapshot of average properties, others give information about the dynamics of the solution.Nuclear magnetic resonance (NMR)
Ions for which the water-exchange rate is slow on the NMR time-scale give separate peaks for molecules in the first solvation shell and for other water molecules. The solvation number is obtained as a ratio of peak areas. Here it refers to the number of water molecules in the first solvation shell. Molecules in the second solvation shell exchange rapidly with solvent molecules, giving rise to a small change in the chemical shift value of un-coordinated water molecules from that of water itself. The main disadvantage of this method is that it requires fairly concentrated solutions, with the associated risk ofion-pair
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each ...
formation with the anion.
X-ray diffraction (XRD)
A solution containing an aqua ion does not have the long-range order that would be present in a crystal containing the same ion, but there is short-range order. X-ray diffraction on solutions yields aradial distribution function
In statistical mechanics, the radial distribution function, (or pair correlation function) g(r) in a system of particles (atoms, molecules, colloids, etc.), describes how density varies as a function of distance from a reference particle.
If ...
from which the coordination number of the metal ion and metal-oxygen distance may be derived. With aqua ions of high charge some information is obtained about the second solvation shell.
This technique requires the use of relatively concentrated solutions. X-rays are scatted by electrons, so scattering power increases with atomic number. This makes hydrogen atoms all but invisible to X-ray scattering.
Large angle X-ray scattering has been used to characterize the second solvation shell with trivalent ions such as Cr3+ and Rh3+. The second hydration shell of Cr3+ was found to have molecules at an average distance of . This implies that every molecule in the first hydration shell is hydrogen bonded to two molecules in the second shell.
Neutron diffraction
Diffraction by neutrons also give aradial distribution function
In statistical mechanics, the radial distribution function, (or pair correlation function) g(r) in a system of particles (atoms, molecules, colloids, etc.), describes how density varies as a function of distance from a reference particle.
If ...
. In contrast to X-ray diffraction, neutrons are scatted by nuclei and there is no relationship with atomic number. Indeed, use can be made of the fact that different isotopes of the same element can have widely different scattering powers. In a classic experiment, measurements were made on four nickel chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for che ...
solutions using the combinations of 58Ni, 60Ni, 35Cl and 37Cl isotopes to yield a very detailed picture of cation and anion solvation. Data for a number of metal salts show some dependence on the salt concentration.
: †Figures in brackets are standard deviation
In statistics, the standard deviation is a measure of the amount of variation or dispersion of a set of values. A low standard deviation indicates that the values tend to be close to the mean (also called the expected value) of the set, whil ...
s on the last significant figure of the value.‡ angle between a M-OH2 bond and the plane of the water molecule.
Most of these data refer to concentrated solutions in which there are very few water molecules that are not in the primary hydration spheres of the cation or anion, which may account for some of the variation of solvation number with concentration even if there is no contact ion pairing. The angle θ gives the angle of tilt of the water molecules relative to a plane in the aqua ion. This angle is affected by the hydrogen bonds formed between water molecules in the primary and secondary solvation shells.
The measured solvation number is a time-averaged value for the solution as a whole. When a measured primary solvation number is fractional there are two or more species with integral solvation numbers present in equilibrium with each other. This also applies to solvation numbers that are integral numbers, within experimental error. For example, the solvation number of 5.5 for a lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
solution could be interpreted as being due to presence of two different aqua ions with equal concentrations.
: i(H2O)6sup>+ i(H2O)5sup>+ + H2O
Another possibility is that there is interaction between a solvated cation and an anion, forming an ion pair
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each o ...
. This is particularly relevant when measurements are made on concentrated salt solutions. For example, a solvation number of 3 for a lithium chloride solution could be interpreted as being due to the equilibrium
: i(H2O)4sup>+ + Cl− i(H2O)3Cl+ H2O
lying wholly in favour of the ion pair.
Vibrational spectra
Infrared spectra and Raman spectra can be used to measure the M-O stretching frequency in metal aqua ions. Raman spectroscopy is particularly useful because the Raman spectrum of water is weak whereas the infrared spectrum of water is intense. Interpretation of the vibration frequencies is somewhat complicated by the presence, in octahedral and tetrahedral ions, of two vibrations, a symmetric one measured in the Raman spectrum and an anti-symmetric one, measured in the infrared spectrum. Although the relationship between vibration frequency and force constant is not simple, the general conclusion that can be taken from these data is that the strength of the M-O bond increases with increasing ionic charge and decreasing ionic size. The M-O stretching frequency of an aqua ion in solution may be compared with its counterpart in a crystal of known structure. If the frequencies are very similar it can be concluded that the coordination number of the metal ion is the same in solution as it is in a compound in the solid state.Dynamic methods
Data such asconductivity
Conductivity may refer to:
*Electrical conductivity, a measure of a material's ability to conduct an electric current
**Conductivity (electrolytic), the electrical conductivity of an electrolyte in solution
** Ionic conductivity (solid state), ele ...
, electrical mobility
Electrical mobility is the ability of charged particles (such as electrons or protons) to move through a medium in response to an electric field that is pulling them. The separation of ions according to their mobility in gas phase is called ion ...
and diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical ...
relate to the movement of ions through a solution. When an ion moves through a solution it tends to take both first and second solvation shells with it. Hence solvation numbers measured from dynamic properties tend to be much higher that those obtained from static properties.
:
Solvation numbers and structures
Hydrogen
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
is not a metal, but like them it tends to lose its valence electron in chemical reactions, forming a cation H+. In aqueous solution, this immediately attaches itself to a water molecule, forming a species generally symbolised as H3O+ (sometimes loosely written H+). Such hydration forms cations that can in essence be considered as (OH2)''n''sup>+.
The solvation of H+ in water is not fully characterised and many different structures have been suggested. Two well-known structures are the ''Zundel cation'' and the ''Eigen cation''. The Eigen solvation structure has the hydronium ion at the center of an complex in which the hydronium is strongly hydrogen-bonded to three neighbouring water molecules. In the Zundel complex the proton is shared equally by two water molecules in a symmetric hydrogen bond
A symmetric hydrogen bond is a special type of hydrogen bond in which the proton is spaced exactly halfway between two identical atoms. The strength of the bond to each of those atoms is equal. It is an example of a 3-center 4-electron bond. This ...
.
Alkali metals
The hydratedlithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense soli ...
cation in water is probably tetrahedral and four-coordinated. There are most probably six water molecules in the primary solvation sphere of the octahedral sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
ion. Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmos ...
is seven-coordinate, and rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
and caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
are probably eight-coordinate square antiprismatic. No data is available for francium
Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22&n ...
.
Alkaline earth metals
:§ Values extrapolated from data for solid-state crystal structures Theberyllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
cation e(H2O)4sup>2+ has a very well-defined primary solvation shell with a tetrahedral BeO4 core.Richens, p. 129. For magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, g(H2O)6sup>2+ is also a well-characterized species, with an octahedral MgO6 core. The situation for calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
is more complicated. Neutron diffraction data gave a solvation number for calcium chloride, CaCl2, which is strongly dependent on concentration: at 1 mol·dm−3, decreasing to at 2.8 mol·dm−3. The enthalpy of solvation decreases with increasing ionic radius. Various solid hydrates are known with 8-coordination in square antiprism
In geometry, the square antiprism is the second in an infinite family of antiprisms formed by an even-numbered sequence of triangle sides closed by two polygon caps. It is also known as an ''anticube''.
If all its faces are regular, it is a sem ...
and dodecahedral
In geometry, a dodecahedron (Greek , from ''dōdeka'' "twelve" + ''hédra'' "base", "seat" or "face") or duodecahedron is any polyhedron with twelve flat faces. The most familiar dodecahedron is the regular dodecahedron with regular pentagon ...
geometry. In water, calcium and strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is e ...
are most probably eight-coordinate square antiprismatic (although seven-coordination for calcium cannot presently be excluded). Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
is not as well-studied: it seems to have a coordination number of either eight or nine. Theoretical simulation of radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
suggests that its aqua cation is ten-coordinate.
Group 3 metals, lanthanides and actinides

Scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
(III) and yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
(III) are both eight-coordinate, but have different structures: scandium has an unusual dicapped triangular prismatic structure (with one cap location empty), while yttrium is square antiprismatic. Lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
(III) is tricapped triangular prismatic, but has a significant water deficit: one of the capping water molecules is significantly closer to the lutetium than the remaining ones and the average coordination number is only 8.2 rather than 9. Based on its ionic radius, lawrencium
Lawrencium is a synthetic chemical element with the symbol Lr (formerly Lw) and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radio ...
(III) is probably nine-coordinate tricapped triangular prismatic with no water deficit.
The trivalent lanthanide ions decrease steadily in size from lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between l ...
to lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
, an effect known as the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
. From lanthanum to dysprosium
Dysprosium is the chemical element with the symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanide ...
, the coordination number is maintained at 9 with a tricapped trigonal prismatic structure, although starting from samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samar ...
the capping water molecules are no longer equally strongly bounded. A water deficit then appears for holmium
Holmium is a chemical element with the symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like a lot of oth ...
through lutetium with the average coordination number dropping to 8.2 at lutetium(III). The configuration is maintained despite the small size of the cations and the water deficit, probably due to strong hydrogen bonding. Europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lan ...
(II) is seven-coordinate, and cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
(IV) is hydrolysed to the oxygen-bridged dimer H2O)7Ce–O–Ce(OH2)7sup>6+.
Actinium
Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance ...
(III) is eleven-coordinate in aqueous solution. Thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
(IV) is nine-coordinate tricapped trigonal prismatic, and it is assumed that the same is true for the other actinide(IV) cations in aqueous solutions (as that is also their solid-state configuration). Studies on coordination number and/or structure for actinides(III) to date stretch only to californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding c ...
. However, since lawrencium(III) has a similar ionic radius to dysprosium(III), it is likely that uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
(III) through lawrencium(III) are all nine-coordinate tricapped triangular prismatic with the capping positions fully occupied. No data is available for fermium(II), mendelevium
Mendelevium is a synthetic element with the symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macrosco ...
(II), or nobelium
Nobelium is a synthetic chemical element with the symbol No and atomic number 102. It is named in honor of Alfred Nobel, the inventor of dynamite and benefactor of science. A radioactive metal, it is the tenth transuranic element and is the penul ...
(II).
Group 4-12 metals
 The ions of these metals in the +2 and +3
The ions of these metals in the +2 and +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s have a solvation number of 6. All have a regular octahedral structure except the aqua ions of chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
(II) and copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
(II) which are subject to Jahn-Teller distortion. In the copper case the two axial Cu−O distances are 238 pm, whereas the four equatorial Cu−O distances are 195 pm in the solid state. However, it is unclear whether Cu2+ has a solvation number of 5 or 6 in aqueous solution, with conflicting experimental reports. The structure of cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
(III) in aqueous solution has not been determined. Copper(I) is estimated to be four-coordinate tetrahedral.
A solvation number of 6 with an octahedral structure is well established for zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
(II) and cadmium
Cadmium is a chemical element with the Symbol (chemistry), symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Li ...
(II) in dilute solutions. In concentrated solutions the Zn2+ ion may adopt a 4-coordinate, tetrahedral, structure, but the evidence is not conclusive because of the possibility of ion pairing and/or hydrolysis. The solvation number of mercury(II) is most likely to be 6. Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
(II) is six-coordinate octahedral, but cadmium
Cadmium is a chemical element with the Symbol (chemistry), symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Li ...
(II) may be in equilibrium between six- and seven-coordination. Mercury(II) is a pseudo-Jahn-Teller-distorted octahedron. The bis aqua structure of the mercury(I) ion, H2O)-Hg-Hg-(OH2)sup>+, found in solid compounds, is not the same as that found in solution which involves three water molecules coordinated to each mercury completing a distorted tetrahedral arrangement. Another aqua species in which there is a metal-metal bond is the molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
(II) species formulated as H2O)4Mo≣Mo(H2O)4sup>4+. Each molybdenum is surrounded by four water molecules in a square-planar arrangement, in a structure similar to that of the known structure of the chloro complex o2Cl8sup>4−.
There are a few divalent and trivalent aqua ions of transition metals in the second and third transition series: ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
(II) and (III), rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
(III), and iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density o ...
(III), all octahedral. (Ruthenium and iridium structures have only been examined in the solid state, but it is assumed that they are the same in aqueous solution.) Molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
(III) is questionable (and may be strongly hydrolyzed in aqueous solution), and molybdenum(II) dimerises with each molybdenum binding four water molecules. Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
(II) and platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
(II) aqua ions were originally thought to be square planar, but are actually strongly tetragonally elongated square-pyramidal or octahedral with the extra one or two water molecules extremely loosely bound. The structure of silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
(I) is disputed: it may be two-coordinate, or it may be four-coordinate with two extra very loosely bound water molecules. Gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
(III) is four-coordinate square planar in the solid state, and it is assumed to have the same structure in aqueous solution. Distortion occurs for low-coordinate metals with strong covalent tendencies due to the second-order Jahn-Teller effect. With oxidation state 4, however, the only unhydrolyzed species are the square antiprismatic zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
(IV), r(H2O)8sup>4+, and hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
(IV), f(H2O)8sup>4+, and even they are extremely prone to hydrolysis: such a zirconium cation is only formed in dilute solutions of ZrIV in strong acid. In practice the cationic species encountered of zirconium and hafnium are polynuclear.
Group 13-17 metals
Thealuminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
(III) aqua ion, l(H2O)6sup>3+ is very well characterized in solution and the solid state. The AlO6 core has octahedral symmetry, point group
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every ...
Oh. The aqua ions of gallium
Gallium is a chemical element with the Symbol (chemistry), symbol Ga and atomic number 31. Discovered by France, French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in boron group, group 13 of the periodic table and is similar to ...
(III), indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
(III) and thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
(III) are also six-coordinate octahedral. The coordination geometry of thallium(I) is not experimentally known, but it is likely to be hemidirected with a large gap in the coordination sphere.
Tin(II) is 3-coordinate hemidirected with a very large gap in the coordination sphere of tin(II). The hydration number of lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
(II) is not well-established and could be anywhere from five to seven. In practice these cations tend to be polynuclear. Bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
(III) is eight-coordinate square antiprismatic in aqueous solution, though in the solid state it is nine-coordinate tricapped triangular prismatic.
The aqua ions of elements near the metal–nonmetal dividing line are very easily hydrolyzed and cannot be easily studied by experiment. There is some evidence that germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors ...
(II) aqua ions can form in perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueo ...
media. However, this is readily oxidised to germanium(IV).Richens, p. 152–4 Antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient ti ...
(III) aqua ions may also exist in dilute solutions of antimony(III) in concentrated acids, but there is no direct evidence of this. Quantum mechanical calculations suggest that germanium(II) and antimony(III) aqua ions should exist and would show extreme distortion of the first coordination sphere due to the high charge density and the stereochemically active lone pairs. For germanium(II), the first shell is calculated to usually have a solvation number of 6, but numbers 4–7 are also possible and the shell splits into two with differing distances from the central Ge2+. Similar investigations for antimony(III) reveal a solvation number of 8, with the first coordination sphere splitting into two hydration hemispheres with 4 water molecules each. Calculations for polonium
Polonium is a chemical element with the symbol Po and atomic number 84. Polonium is a chalcogen. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character ...
(IV) indicate a solvation number between 8 and 9.
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, b ...
(III) is calculated to form hydrolyzed species only. The stable cationic arsenic(III) species in water is calculated to be s(OH)2sup>+. In oxidation state +4, only hydrolyzed species are expected for germanium(IV), tin(IV), and lead(IV). Cationic tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionall ...
(IV) appears to be e(OH)3sup>+.
A cationic astatine
Astatine is a chemical element with the symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-live ...
(I) species is inferred from trace-scale experiments in acidic solutions, but it is not clear whether it is an aqua cation or not. Some authors have suggested that it could be a symmetric diaqua complex t(H2O)2sup>+, but electromigration evidence suggests that it is instead protonated hypoastatous acid 2OAtsup>+, showing analogy to iodine.
Although the structures for thallium(I), germanium(II), tin(II), lead(II), and antimony(III) are affected by the lone pairs, this is not so for bismuth(III).
Oxo-aqua-cations
Some elements in oxidation states higher than 3 form stable, aquated, oxo ions. Well known examples are the vanadyl(IV) anduranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula . It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may ...
(VI) ions. They can be viewed as particularly stable hydrolysis products in a hypothetical reaction such as
: (H2O)6sup>4+ → O(H2O)5sup>2+ + 2H+
The vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pass ...
has a distorted octahedral environment ( point group C4v) of one oxide ion and 5 water molecules. Titanyl, TiO2+, has a similar structure. Vanadium(V) is believed to exist as the dioxo-ion O2(H2O)4sup>+ at pH less than 2, but the evidence for this ion depends on the formation of complexes, such as oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
complexes which have been shown to have the unit, with ''cis''-VO bonds, in the solid state. The chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
(IV) ion rO(H2O)5sup>2+, similar to the vanadium ion has been proposed on the basis of indirect evidence.
The uranyl ion, , has a ''trans'' structure. The aqua ion (aq) has five water molecules in the plane perpendicular to the O-U-O axis in a pentagonal bipyramid
In geometry, the pentagonal bipyramid (or dipyramid) is third of the infinite set of face-transitive bipyramids, and the 13th Johnson solid (). Each bipyramid is the dual of a uniform prism.
Although it is face-transitive, it is not a Plato ...
structure, point group D5h. Neptunyl and plutonyl have the same structure. Nothing is known of actinide(V) structures.
Thermodynamics
The main goal of thermodynamics in this context is to derive estimates of single-ion thermodynamic quantities such as hydrationenthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
and hydration entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
. These quantities relate to the reaction
:Mz+ (gas) + solvent → Mz+ (in solution)
The enthalpy for this reaction is not directly measurable, because all measurements use salt solutions that contain both cation and anion. Most experimental measurements relate to the heat evolved when a salt dissolves in water, which gives the sum of cation and anion solvation enthalpies. Then, by considering the data for different anions with the same cation and different cations with the same anion, single ion values relative to an arbitrary zero, are derived.

 :
Other values include Zn2+ -2044.3, Cd2+ -1805.8 and Ag+ -475.3 kJ mol−1.
There is an excellent linear correlation between hydration enthalpy and the ratio of charge squared, z2, to M-O distance, reff.
:
Values for transition metals are affected by
:
Other values include Zn2+ -2044.3, Cd2+ -1805.8 and Ag+ -475.3 kJ mol−1.
There is an excellent linear correlation between hydration enthalpy and the ratio of charge squared, z2, to M-O distance, reff.
:
Values for transition metals are affected by crystal field Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually ''d'' or ''f'' orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors). This theory has been used ...
stabilization. The general trend is shown by the magenta line which passes through Ca2+, Mn2+ and Zn2+, for which there is no stabilization in an octahedral crystal field. Hydration energy increases as size decreases. Crystal field splitting confers extra stability on the aqua ion. The maximum crystal field stabilization energy occurs at Ni2+. The agreement of the hydration enthalpies with predictions provided one basis for the general acceptance of crystal field theory.
The hydration enthalpies of the trivalent lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
ions show an increasingly negative values at atomic number increases, in line with the decrease in ionic radius known as the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
.
Single ion hydration entropy can be derived. Values are shown in the following table. The more negative the value, the more there is ordering in forming the aqua ion. It is notable that the heavy alkali metals have rather small entropy values which suggests that both the first and second solvation shells are somewhat indistinct.
:
Hydrolysis of aqua ions
There are two ways of looking at an equilibrium involving hydrolysis of an aqua ion. Considering the dissociation equilibrium : (H2O)nsup>z+ - H+ (H2O)n-1(OH)sup>(z-1)+ the activity of the hydrolysis product, omitting the water molecules, is given by : The alternative is to write the equilibrium as a complexation or substitution reaction : (H2O)nsup>z+ +OH− : (H2O)n-1(OH)sup>(z-1)+ + H2O In which case : The concentration of hydrogen and hydroxide ions are related by theself-ionization of water
The self-ionization of water (also autoionization of water, and autodissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen ...
, Kw = so the two equilibrium constants are related as
:
In practice the first definition is more useful because equilibrium constants are determined from measurements of hydrogen ion concentrations. In general,
:
charges are omitted for the sake of generality and activities have been replaced by concentrations. are cumulative hydrolysis constants.
Modeling the hydrolysis reactions that occur in solution is usually based on the determination of equilibrium constants Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,
:K=\frac
it is implied that the activity quotient is constant. For this assumption to be vali ...
from potentiometric (pH) titration data. The process is far from straightforward for a variety of reasons. Sometimes the species in solution can be precipitated as salts and their structure confirmed by X-ray crystallography. In other cases, precipitated salts bear no relation to what is postulated to be in solution, because a particular crystalline substances may have both low solubility and very low concentration in the solutions.
First hydrolysis constant
The logarithm of hydrolysis constant, K1,-1, for the removal of one proton from an aqua ion : (H2O)nsup>z+ - H+ (H2O)n-1(OH)sup>(z-1)+ : [M(OH)sup>{(z-1)+_.html" ;"title="(OH).html" ;"title="[M(OH)">[M(OH)sup>{(z-1)+ ">(OH).html" ;"title="[M(OH)">[M(OH)sup>{(z-1)+ = K1,-1 [Mz+] [H+] −1 shows a linear relationship with the ratio of charge to M-O distance, z/d. Ions fall into four groups. The slope of the straight line is the same for all groups, but the intercept, A, is different.Baes&Mesmer, p407 :{, class="wikitable" , +log K1,-1 = A + 11.0 z/d !cation, , A , - , Mg2+, Ca2+, Sr2+, Ba2+Al3+, Y3+, La3+, , , - , Li+, Na+, K+
Be2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+
Sc3+, Ti3+, V3+, Cr3+, Fe3+, Rh3+, Ga3+, In3+
Ce4+, Th4+, Pa4+, U4+, Np4+, Pu4+, , , , - , Ag+, Tl+
Pb2+
Ti3+, Bi3+, , , , - , Sn2+, Hg2+, Sn2+, Pd2+ , , ca. 12 The cations most resistant to hydrolysis for their size and charge are hard pre-transition metal ions or lanthanide ions. The slightly less resistant group includes the transition metal ions. The third group contains mostly soft ions ion of post-transition metals. The ions which show the strongest tendency to hydrolyze for their charge and size are Pd2+, Sn2+ and Hg2+. The standard enthalpy change for the first hydrolysis step is generally not very different from that of the dissociation of pure water. Consequently, the standard enthalpy change for the substitution reaction : (H2O)nsup>z+ +OH− : (H2O)n-1(OH)sup>(z-1)+ + H2O is close to zero. This is typical of reactions between a hard cation and a hard anion, such as the hydroxide ion. It means that the standard entropy charge is the major contributor to the standard free energy change and hence the equilibrium constant. : The change in ionic charge is responsible for the effect as the aqua ion has a greater ordering effect on the solution than the less highly charged hydroxo complex.
Multiple hydrolysis reactions

 The hydrolysis of beryllium shows many of the characteristics typical of multiple hydrolysis reactions. The concentrations of various species, including polynuclear species with bridging hydroxide ions, change as a function of pH up to the precipitation of an insoluble hydroxide. Beryllium hydrolysis is unusual in that the concentration of e(H2O)3(OH)sup>+ is too low to be measured. Instead a trimer ([Be3(H2O)6(OH3))3+ is formed, whose structure has been confirmed in solid salts. The formation of polynuclear species is driven by the reduction in charge density within the molecule as a whole. The local environment of the beryllium ions approximates to [Be(H2O)2(OH)2]+. The reduction in effective charge releases free energy in the form of a decrease of the entropy of ordering at the charge centers.
:{, class="wikitable"
, +Some polynuclear hydrolysis products
! Species formula!! cations!! ! scope="col" width="300" , structure
, -
, M2(OH)+, , Be2+, Mn2+, Co2+, Ni2+
The hydrolysis of beryllium shows many of the characteristics typical of multiple hydrolysis reactions. The concentrations of various species, including polynuclear species with bridging hydroxide ions, change as a function of pH up to the precipitation of an insoluble hydroxide. Beryllium hydrolysis is unusual in that the concentration of e(H2O)3(OH)sup>+ is too low to be measured. Instead a trimer ([Be3(H2O)6(OH3))3+ is formed, whose structure has been confirmed in solid salts. The formation of polynuclear species is driven by the reduction in charge density within the molecule as a whole. The local environment of the beryllium ions approximates to [Be(H2O)2(OH)2]+. The reduction in effective charge releases free energy in the form of a decrease of the entropy of ordering at the charge centers.
:{, class="wikitable"
, +Some polynuclear hydrolysis products
! Species formula!! cations!! ! scope="col" width="300" , structure
, -
, M2(OH)+, , Be2+, Mn2+, Co2+, Ni2+ Zn2+, Cd2+, Hg2+, Pb2+ , , single hydroxide bridge between two cations , - , M2(OH) , , Cu2+, Sn2+
Al3+, Sc3+, Ln3+, Ti3+, Cr3+
Th4+
VO2+, , , , , double hydroxide bridge between two cations , - , , , Be2+, Hg2+ , , six-membered ring with alternate Mn+ and OH− groups , - , (OH) , , Sn2+, Pb2+
Al3+, Cr3+, Fe3+, In3+ , , Cube with alternate vertices of Mn+ and OH− groups, one vertex missing , - , , , Mg2+, Co2+, Ni2+, Cd2+, Pb2+ , , Cube with alternate vertices of Mn+ and OH− groups , - , , , Zr4+, Th4+ , , Square of Mn+ ions with double hydroxide bridges on each side of the square The hydrolysis product of
aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
formulated as l13O4(OH)24(H2O)12sup>7+ is very well characterized and may be present in nature in water at pH ca. 5.4.
The overall reaction for the loss of two protons from an aqua ion can be written as
: (H2O)nsup>z+ - 2 H+ (H2O)n-2(OH)2sup>(z-2)+
However, the equilibrium constant for the loss of two protons applies equally well to the equilibrium
: (H2O)nsup>z+ - 2 H+ O(H2O)n-2sup>(z-2)+ + H2O
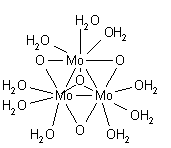
because the concentration of water is assumed to be constant. This applies in general: any equilibrium constant is equally valid for a product with an oxide ion as for the product with two hydroxyl ions. The two possibilities can only be distinguished by determining the structure of a salt in the solid state. Oxo bridges tend to occur when the metal oxidation state is high. An example is provided by the molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
(IV) complex o3O4(H2O)9sup>4+ in which there is a triangle of molybdenum atoms joined by σ- bonds with an oxide bridge on each edge of the triangle and a fourth oxide which bridges to all three Mo atoms.
Oxyanions
There are very few oxo-aqua ions of metals in the oxidation state +5 or higher. Rather, the species found in aqueous solution are monomeric and polymeric oxyanions. Oxyanions can be viewed as the end products of hydrolysis, in which there are no water molecules attached to the metal, only oxide ions.Exchange kinetics
A water molecule in the first solvation shell of an aqua ion may exchange places with a water molecule in the bulk solvent. It is usually assumed that therate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
is a dissociation reaction.
: (H2O)nsup>z+ → (H2O)n-1sup>z+* + H2O
The * symbol signifies that this is the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
in a chemical reaction. The rate of this reaction is proportional to the concentration of the aqua ion,
: