List of viscosities on:
[Wikipedia]
[Google]
[Amazon]
Dynamic viscosity is a material property which describes the resistance of a fluid to shearing flows. It corresponds roughly to the intuitive notion of a fluid's 'thickness'. For instance,
 All values are given at 1 bar (approximately equal to
All values are given at 1 bar (approximately equal to
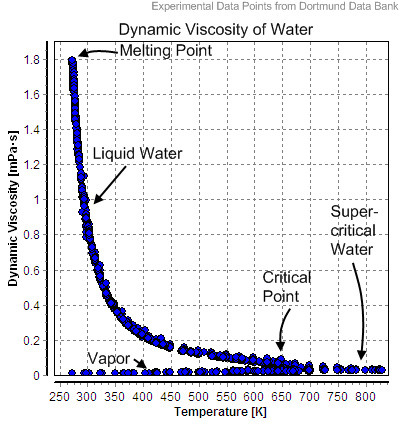 In the following table, the temperature is given in
In the following table, the temperature is given in
honey
Honey is a sweet and viscous substance made by several bees, the best-known of which are honey bees. Honey is made and stored to nourish bee colonies. Bees produce honey by gathering and then refining the sugary secretions of plants (primar ...
has
a much higher viscosity than water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
. Viscosity is measured using a viscometer
A viscometer (also called viscosimeter) is an instrument used to measure the viscosity of a fluid. For liquids with viscosities which vary with flow conditions, an instrument called a rheometer is used. Thus, a rheometer can be considered as a spe ...
. Measured values span several orders
of magnitude. Of all fluids, gases have the lowest viscosities, and thick liquids have the highest.
The values listed in this article are representative estimates only, as they do not account for measurement uncertainties, variability in material definitions, or non-Newtonian behavior.
Kinematic viscosity is dynamic viscosity divided by fluid density. This page lists only dynamic viscosity.
Units and conversion factors
For dynamic viscosity, the SI unit is Pascal-second. In engineering, the unit is usually Poise or centiPoise, with 1 Poise = 0.1 Pascal-second, and 1 centiPoise = 0.01 Poise. For kinematic viscosity, the SI unit is m^2/s. In engineering, the unit is usually Stoke or centiStoke, with 1 Stoke = 0.0001 m^2/s, and 1 centiStoke = 0.01 Stoke. For liquid, the dynamic viscosity is usually in the range of 0.001 to 1 Pascal-second, or 1 to 1000 centiPoise. The density is usually on the order of 1000 kg/m^3, i.e. that of water. Consequently, if a liquid has dynamic viscosity of n centiPoise, and its density is not too different from that of water, then its kinematic viscosity is around n centiStokes. For gas, the dynamic viscosity is usually in the range of 10 to 20 microPascal-seconds, or 0.01 to 0.02 centiPoise. The density is usually on the order of 0.5 to 5 kg/m^3. Consequently, its kinematic viscosity is around 2 to 40 centiStokes.Viscosities at or near standard conditions
Here "standard conditions" refers to temperatures of 25 °C and pressures of 1 atmosphere. Where data points are unavailable for 25 °C or 1 atmosphere, values are given at a nearby temperature/pressure. The temperatures corresponding to each data point are stated explicitly. By contrast, pressure is omitted since gaseous viscosity depends only weakly on it.Gases
Noble gases
The simple structure ofnoble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
molecules makes them amenable to accurate theoretical treatment. For this reason, measured viscosities of the noble gases serve as important tests of the kinetic-molecular theory of transport processes in gases (see Chapman–Enskog theory
Chapman–Enskog theory provides a framework in which equations of hydrodynamics for a gas can be derived from the Boltzmann equation. The technique justifies the otherwise phenomenological constitutive relations appearing in hydrodynamical descri ...
). One of the key predictions of the theory is the following relationship between viscosity , thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
, and specific heat :
:
where is a constant which in general depends on the details of intermolecular interactions, but for spherically symmetric molecules is very close to .
This prediction is reasonably well-verified by experiments, as the following table shows. Indeed, the relation provides a viable means for obtaining thermal conductivities of gases since these are more difficult to measure directly than viscosity.
Diatomic elements
Hydrocarbons
Organohalides
Other gases
Liquids
n-Alkanes
Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. More dramatically, a long-chain hydrocarbon likesqualene
Squalene is an organic compound. It is a triterpenoid with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as ''Squalus'' is a genus of sharks). A ...
(C30H62) has a viscosity an order of magnitude larger than the shorter n-alkanes (roughly 31 mPa·s at 25 °C). This is also the reason oils tend to be highly viscous, since they are usually composed of long-chain hydrocarbons.
1-Chloroalkanes
Other halocarbons
Alkenes
Other liquids
Aqueous solutions
The viscosity of an aqueous solution can either increase or decrease with concentration depending on the solute and the range of concentration. For instance, the table below shows that viscosity increases monotonically with concentration for sodium chloride andcalcium chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Ca ...
, but decreases for potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
and cesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
(the latter up to 30% mass percentage, after which viscosity increases).
The increase in viscosity for sucrose solutions is particularly dramatic, and explains in part the common experience of sugar water being "sticky".
Substances of variable composition
Viscosities under nonstandard conditions
Gases
atmospheric pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibars, ...
).
Liquids (including liquid metals)
 In the following table, the temperature is given in
In the following table, the temperature is given in kelvins
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phy ...
.
Solids
References
{{ReflistViscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...