Lever rule on:
[Wikipedia]
[Google]
[Amazon]
In

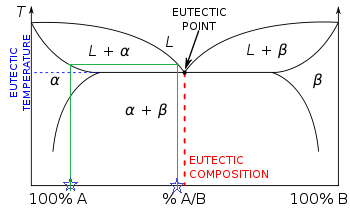 There is now more than one two-phase region. The tie line drawn is from the solid alpha to the liquid and by dropping a vertical line down at these points the mass fraction of each phase is directly read off the graph, that is the mass fraction in the x axis element. The same equations can be used to find the mass fraction of alloy in each of the phases, i.e. wl is the mass fraction of the whole sample in the liquid phase.
There is now more than one two-phase region. The tie line drawn is from the solid alpha to the liquid and by dropping a vertical line down at these points the mass fraction of each phase is directly read off the graph, that is the mass fraction in the x axis element. The same equations can be used to find the mass fraction of alloy in each of the phases, i.e. wl is the mass fraction of the whole sample in the liquid phase.
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, the lever rule is a formula used to determine the mole fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the to ...
(''xi'') or the mass fraction (''wi'') of each phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
*Phase space, a mathematica ...
of a binary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two values (0 and 1) for each digit
* Binary function, a function that takes two arguments
* Binary operation, a mathematical op ...
equilibrium phase diagram. It can be used to determine the fraction of liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
and solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
phases for a given binary composition and temperature that is between the liquidus
While chemically pure materials have a single melting point, chemical mixtures often partially melt at the temperature known as the solidus (''T''S or ''T''sol), and fully melt at the higher liquidus temperature (''T''L or ''T''liq). The solidus ...
and solidus
Solidus (Latin for "solid") may refer to:
* Solidus (coin)
The ''solidus'' (Latin 'solid'; : ''solidi'') or ''nomisma'' () was a highly pure gold coin issued in the Later Roman Empire and Byzantine Empire. It was introduced in the early ...
line.
In an alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
or a mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method. It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proporti ...
with two phases, α and β, which themselves contain two element
Element or elements may refer to:
Science
* Chemical element, a pure substance of one type of atom
* Heating element, a device that generates heat by electrical resistance
* Orbital elements, parameters required to identify a specific orbit of o ...
s, A and B, the lever rule states that the mass fraction of the α phase is
:
where
* is the mass fraction of element B in the α phase
* is the mass fraction of element B in the β phase
* is the mass fraction of element B in the entire alloy or mixture
all at some fixed temperature or pressure.
Derivation
Suppose an alloy at an equilibrium temperature ''T'' consists of mass fraction of element B. Suppose also that at temperature ''T'' the alloy consists of two phases, α and β, for which the α consists of , and β consists of . Let the mass of the α phase in the alloy be so that the mass of the β phase is , where is the total mass of the alloy. By definition, then, the mass of element B in the α phase is , while the mass of element B in the β phase is . Together these two quantities sum to the total mass of element B in the alloy, which is given by . Therefore, : By rearranging, one finds that : This final fraction is the mass fraction of the α phase in the alloy.Calculations
Binary phase diagrams
Before any calculations can be made, a ''tie line'' is drawn on the phase diagram to determine the mass fraction of each element; on the phase diagram to the right it isline segment
In geometry, a line segment is a part of a line (mathematics), straight line that is bounded by two distinct endpoints (its extreme points), and contains every Point (geometry), point on the line that is between its endpoints. It is a special c ...
LS. This tie line is drawn horizontally at the composition's temperature from one phase to another (here the liquid to the solid). The mass fraction of element B at the liquidus is given by ''w''Bl (represented as ''w''l in this diagram) and the mass fraction of element B at the solidus is given by ''w''Bs (represented as ''w''s in this diagram). The mass fraction of solid and liquid can then be calculated using the following lever rule equations:
:
:
where ''w''B is the mass fraction of element B for the given composition (represented as ''w''o in this diagram).
The numerator of each equation is the original composition that we are interested in is +/- the opposite ''lever arm''. That is if you want the mass fraction of solid then take the difference between the liquid composition and the original composition. And then the denominator is the overall length of the arm so the difference between the solid and liquid compositions. If you're having difficulty realising why this is so, try visualising the composition when ''w''o approaches ''w''l. Then the liquid concentration will start increasing.
Eutectic phase diagrams
 There is now more than one two-phase region. The tie line drawn is from the solid alpha to the liquid and by dropping a vertical line down at these points the mass fraction of each phase is directly read off the graph, that is the mass fraction in the x axis element. The same equations can be used to find the mass fraction of alloy in each of the phases, i.e. wl is the mass fraction of the whole sample in the liquid phase.
There is now more than one two-phase region. The tie line drawn is from the solid alpha to the liquid and by dropping a vertical line down at these points the mass fraction of each phase is directly read off the graph, that is the mass fraction in the x axis element. The same equations can be used to find the mass fraction of alloy in each of the phases, i.e. wl is the mass fraction of the whole sample in the liquid phase.
References
{{reflist Metallurgy Phase transitions Materials science Charts Diagrams