Laron syndrome on:
[Wikipedia]
[Google]
[Amazon]
Laron syndrome (LS), also known as growth hormone insensitivity or growth hormone receptor deficiency (GHRD), is an

 Under normal circumstances in humans, growth hormone (GH) is released in a pulsatile fashion from cells known as somatotrophs in the anterior pituitary gland. These pulses of GH are regulated by cells in the
Under normal circumstances in humans, growth hormone (GH) is released in a pulsatile fashion from cells known as somatotrophs in the anterior pituitary gland. These pulses of GH are regulated by cells in the
autosomal
An autosome is any chromosome that is not a sex chromosome. The members of an autosome pair in a diploid cell have the same morphology, unlike those in allosomal (sex chromosome) pairs, which may have different structures. The DNA in autosom ...
recessive
In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ...
disorder characterized by a lack of insulin-like growth factor 1
Insulin-like growth factor 1 (IGF-1), also called somatomedin C, is a hormone similar in molecular structure to insulin which plays an important role in childhood growth, and has anabolic effects in adults.
IGF-1 is a protein that in humans ...
(IGF-1; somatomedin) production in response to growth hormone
Growth hormone (GH) or somatotropin, also known as human growth hormone (hGH or HGH) in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in h ...
(GH; hGH; somatotropin). It is usually caused by inherited growth hormone receptor
Growth hormone receptor is a protein that in humans is encoded by the ''GHR'' gene. GHR orthologs have been identified in most mammals.
Structure
Growth hormone receptor (GHR) is a transmembrane protein consisting of 620 amino acids. The recep ...
(GHR) mutations.
Affected individuals classically present with short stature
Short stature refers to a height of a human which is below typical. Whether a person is considered short depends on the context. Because of the lack of preciseness, there is often disagreement about the degree of shortness that should be called ' ...
between −4 to −10 standard deviations below median height, obesity, craniofacial abnormalities, micropenis
Micropenis is an unusually small penis. A common criterion is a dorsal (measured on top) penile length of at least 2.5 standard deviations smaller than the mean human penis size (stretched penile length less than 9.3 cm (3.67 in) in adults). ...
, low blood sugar
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose bel ...
, and low serum IGF-1 despite elevated basal serum GH.
LS is a very rare condition with a total of 250 known individuals worldwide. The genetic origins of these individuals have been traced back to Mediterranean, South Asian, and Semitic ancestors, with the latter group comprising the majority of cases. Molecular genetic testing for growth hormone receptor gene mutations confirms the diagnosis of LS, but clinical evaluation may include laboratory analysis of basal GH, IGF-1 and IGFBP levels, GH stimulation testing, and/or GH trial therapy. Treatment options include recombinant IGF-1 (Mecasermin).
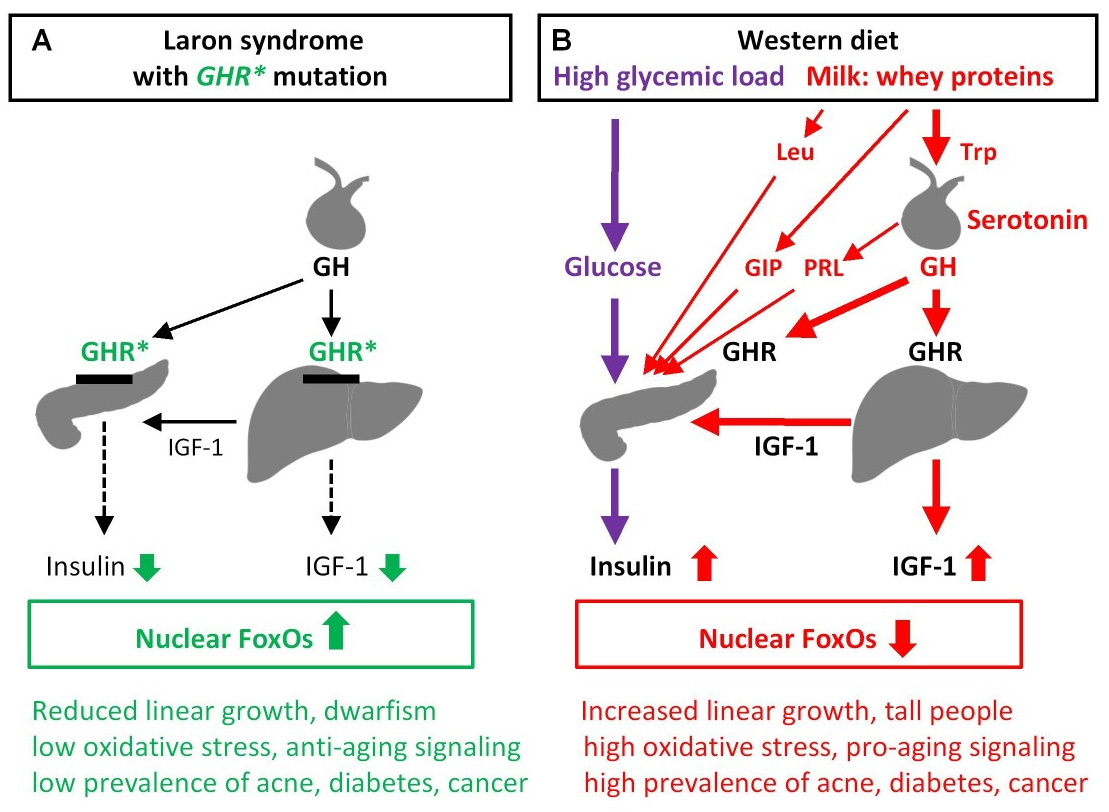
Evidence has suggested that people with Laron syndrome have a reduced risk of developing cancer and diabetes mellitus type II, with a significantly reduced incidence and delayed age of onset of these diseases compared to their unaffected relatives. The molecular mechanisms of increased longevity and protection from age-related disease among people with LS is an area of active investigation.
Presentation
Physical Features
LS is recognized as being part of a spectrum of conditions that affect the Hypothalamic–pituitary–somatotropic axis and cause significant derangements in human growth, development, and metabolism. Along this spectrum of conditions, individuals with LS andgrowth hormone deficiency
Growth hormone deficiency (GHD), or human growth hormone deficiency, is a medical condition resulting from not enough growth hormone (GH). Generally the most noticeable symptom is that an individual attains a short height. Newborns may also prese ...
display short stature
Short stature refers to a height of a human which is below typical. Whether a person is considered short depends on the context. Because of the lack of preciseness, there is often disagreement about the degree of shortness that should be called ' ...
, while individuals with acromegaly and gigantism result in the opposite phenotype
In genetics, the phenotype () is the set of observable characteristics or traits of an organism. The term covers the organism's morphology or physical form and structure, its developmental processes, its biochemical and physiological pr ...
of tall stature
Human height or stature is the distance from the bottom of the feet to the top of the head in a human body, standing erect. It is measured using a stadiometer, in centimetres when using the metric system or SI system, or feet and inches when u ...
.
In addition to short stature, other characteristic physical symptoms of LS include: prominent forehead, depressed nasal bridge
The nasal bridge is the upper, bony part of the human nose, which overlies the nasal bones.
Association with epicanthic folds
Low-rooted nasal bridges are closely associated with epicanthic folds. A lower nasal bridge is more likely to cause an ...
, underdevelopment of mandible
In anatomy, the mandible, lower jaw or jawbone is the largest, strongest and lowest bone in the human facial skeleton. It forms the lower jaw and holds the lower teeth in place. The mandible sits beneath the maxilla. It is the only movable bone ...
, truncal obesity
Obesity is a medical condition, sometimes considered a disease, in which excess body fat has accumulated to such an extent that it may negatively affect health. People are classified as obese when their body mass index (BMI)—a person's ...
, and micropenis
Micropenis is an unusually small penis. A common criterion is a dorsal (measured on top) penile length of at least 2.5 standard deviations smaller than the mean human penis size (stretched penile length less than 9.3 cm (3.67 in) in adults). ...
in males. Left untreated, the average height attained by individuals with LS are approximately 4-4.5 feet in women/men respectively. Additional physical features include delayed bone age, hypogonadism
Hypogonadism means diminished functional activity of the gonads—the testes or the ovaries—that may result in diminished production of sex hormones. Low androgen (e.g., testosterone) levels are referred to as hypoandrogenism and low estroge ...
, blue sclera, high-pitched voice, acrohypoplasia, sparse hair growth, and crowded teeth. The breast
The breast is one of two prominences located on the upper ventral region of a primate's torso. Both females and males develop breasts from the same embryological tissues.
In females, it serves as the mammary gland, which produces and sec ...
s of females reach normal size, and in some are large in relation to body size. It has been suggested that hyperprolactinemia
Hyperprolactinaemia is the presence of abnormally high levels of prolactin in the blood. Normal levels average to about 13 ng/mL in women, and 5 ng/mL in men, with an upper normal limit of serum prolactin levels being 15-25 ng/mL ...
may contribute to the enlarged breast size. Seizures
An epileptic seizure, informally known as a seizure, is a period of symptoms due to abnormally excessive or synchronous neuronal activity in the brain. Outward effects vary from uncontrolled shaking movements involving much of the body with l ...
are frequently seen secondary to hypoglycemia
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose bel ...
. Some genetic variations decrease intellectual
An intellectual is a person who engages in critical thinking, research, and reflection about the reality of society, and who proposes solutions for the normative problems of society. Coming from the world of culture, either as a creator or a ...
capacity. Laron syndrome patients also do not develop acne, except temporarily during treatment with IGF-1 (if performed).
Pathophysiology

 Under normal circumstances in humans, growth hormone (GH) is released in a pulsatile fashion from cells known as somatotrophs in the anterior pituitary gland. These pulses of GH are regulated by cells in the
Under normal circumstances in humans, growth hormone (GH) is released in a pulsatile fashion from cells known as somatotrophs in the anterior pituitary gland. These pulses of GH are regulated by cells in the hypothalamus
The hypothalamus () is a part of the brain that contains a number of small nuclei with a variety of functions. One of the most important functions is to link the nervous system to the endocrine system via the pituitary gland. The hypothalamu ...
, via the release of growth hormone-releasing hormone (GHRH) into the hypothalamohypophysial system when stimulated by insulin, ghrelin
Ghrelin (; or lenomorelin, INN) is a hormone produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are hi ...
, glucagon, arginine, deep sleep, exercise, fasting, sex hormone
Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effect ...
release during puberty, and a host of other factors. GH release is inhibited by somatostatin (GHIH), IGF-1, hyperglycemia, and glucocorticoids
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebr ...
. Once released, the GH molecules travel through the bloodstream and eventually bind to GH receptors on the surface of cells composing bodily organs and tissues. One major site of action for GH is in the liver, where it stimulates gluconeogenesis and the release of IGF-1 through the JAK-STAT signaling pathway
The JAK-STAT signaling pathway is a chain of interactions between proteins in a cell, and is involved in processes such as immunity, cell division, cell death, and tumour formation. The pathway communicates information from chemical signals ou ...
. IGF-1 promotes growth in a variety of tissues throughout the body, especially bone mineralization, and provides negative feedback on GH release. GH results in increased muscle mass, lipolysis
Lipolysis is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and free fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important ...
, and protein synthesis. Obesity
Obesity is a medical condition, sometimes considered a disease, in which excess body fat has accumulated to such an extent that it may negatively affect health. People are classified as obese when their body mass index (BMI)—a person's ...
and increased adipose tissue
Adipose tissue, body fat, or simply fat is a loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular ...
, especially visceral fat, results in reduced GH secretion. There is a natural age-related decline in the GHRH-stimulated release of GH.
Growth Hormone Receptor Mutations
Molecular genetic investigations have shown that LS is mainly associated with autosomal recessive mutations in thegene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
for the growth hormone receptor
Growth hormone receptor is a protein that in humans is encoded by the ''GHR'' gene. GHR orthologs have been identified in most mammals.
Structure
Growth hormone receptor (GHR) is a transmembrane protein consisting of 620 amino acids. The recep ...
(GHR). These can result in defective hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
binding to the ectodomain or reduced efficiency of dimerization of the receptor after hormone occupancy.
LS is generally classified as "primary" GH insensitivity, which is distinguished from "secondary" GH insensitivity. Primary (congenital/hereditary) GH insensitivity may result from growth hormone receptor defects, as in the case of Laron syndrome, but can also be caused by defective post-receptor signal transduction ( STAT5B), abnormalities of the IGF-1 gene or IGF-1 receptor. Secondary (acquired) GH insensitivity results from antibodies to growth hormone or the growth hormone receptor, as well as poor nutritional status, liver disease
Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.
Signs and symptoms
Some of the si ...
or diabetes mellitus
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ...
. A GHR mutation that results in only partial insensitivity to GH can manifest as a form of idiopathic short stature.
STAT5B
A related condition involving postreceptor insensitivity to growth hormone has been associated with STAT5B.Diagnosis
LS should be suspected in children or adults with distinctive physical features listed above, extremely elevated serum hGH concentrations despite low serum IGF-1 levels. A failure of IGF-1 to increase in response to exogenous hGH (IGF-1 stimulation test) is diagnostic for LS. The gold standard for confirming a diagnosis of LS is to perform agenetic analysis
Genetic analysis is the overall process of studying and researching in fields of science that involve genetics and molecular biology. There are a number of applications that are developed from this research, and these are also considered parts of ...
with PCR to identify the precise molecular defect in the GH receptor gene. Other laboratory abnormalities include GHBP ''(growth hormone binding protein)'' levels being low in cases with mutations in the extracellular domain of the GH receptor and normal in cases with mutations in the intracellular domain. Low serum levels of IGFBP are non-diagnostic for LS.
Treatment
Administration of GH has no effect on IGF-1 production, therefore treatment is mainly by biosynthetic IGF-1. IGF-1 must be taken beforepuberty
Puberty is the process of physical changes through which a child's body matures into an adult body capable of sexual reproduction. It is initiated by hormonal signals from the brain to the gonads: the ovaries in a girl, the testes in a bo ...
to be effective.
The drug product Increlex (mecasermin), developed by the company Tercica
Tercica, Inc., was a biopharmaceutical company based in Brisbane, California, United States. It developed Increlex (mecasermin DNA origininjection), also known as recombinant human Insulin-like Growth Factor-1 ( rhIGF-1). Tercica applied to the ...
, purchased by Ipsen
Ipsen is a French biopharmaceutical company headquartered in Paris, France, with a focus on transformative medicines in three therapeutic areas: oncology, rare disease and neuroscience. Ipsen is one of the world’s top 15 biopharmaceutical com ...
, was approved by the US Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon ...
in August 2005 for replacing IGF-1 in patients who are deficient.
IPLEX (Mecasermin rinfabate) is composed of recombinant human IGF-1 (rhIGF-1) and its binding protein IGFBP-3
Insulin-like growth factor-binding protein 3, also known as IGFBP-3, is a protein that in humans is encoded by the ''IGFBP3'' gene. IGFBP-3 is one of six IGF binding proteins ( IGFBP-1 to IGFBP-6) that have highly conserved structures and bind th ...
. It was approved by the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon ...
(FDA) in 2005 for treatment of primary IGF-1 deficiency or GH gene deletion. Side effects from IPLEX are hypoglycemia. IPLEX's manufacturing company, Insmed, after selling its protein production facility, can no longer develop proteins, thus can no longer manufacture IPLEX as of a statement released in July 2009.
Prognosis
Cancer and Diabetes
It has been reported that people with LS in Ecuador are resistant to cancer and diabetes and are somewhat protected against aging. This is consistent with findings in mice with a defective growth hormone receptor gene. Among the approximately 100 individuals in this population, there were no reported cases of diabetes and one case of cancer. A 2019 study of individuals with isolated growth hormone deficiency (IGHD type 1B) in Itabaianinha County, Brazil demonstrated a phenotype consistent with Laron syndrome. Researchers found that these humans had similarly extendedhealthspan
Life expectancy is a statistical measure of the average time an organism is expected to live, based on the year of its birth, current age, and other demographic factors like sex. The most commonly used measure is life expectancy at birth ...
, with resistance to cancer and attenuated effects of aging, but neither patients with LS nor IGHD experienced an increase in their overall lifespan.
Incidence
The majority of reported cases of Laron syndrome have been in people with Semitic origins, almost all of them beingJews
Jews ( he, יְהוּדִים, , ) or Jewish people are an ethnoreligious group and nation originating from the Israelites Israelite origins and kingdom: "The first act in the long drama of Jewish history is the age of the Israelites""The ...
or assimilated descendants of Jews.
Numerous Laron syndrome patients are found in Israel
Israel (; he, יִשְׂרָאֵל, ; ar, إِسْرَائِيل, ), officially the State of Israel ( he, מְדִינַת יִשְׂרָאֵל, label=none, translit=Medīnat Yīsrāʾēl; ), is a country in Western Asia. It is situated ...
among the country's diverse Jewish population
As of 2020, the world's "core" Jewish population (those identifying as Jews above all else) was estimated at 15 million, 0.2% of the 8 billion worldwide population. This number rises to 18 million with the addition of the "connected" Jewish pop ...
composed of Jews from around the world, as well as patients outside Israel originally from communities of the Jewish diaspora
The Jewish diaspora ( he, תְּפוּצָה, təfūṣā) or exile (Hebrew: ; Yiddish: ) is the dispersion of Israelites or Jews out of their ancient ancestral homeland (the Land of Israel) and their subsequent settlement in other parts of th ...
, such as Egypt
Egypt ( ar, مصر , ), officially the Arab Republic of Egypt, is a transcontinental country spanning the northeast corner of Africa and southwest corner of Asia via a land bridge formed by the Sinai Peninsula. It is bordered by the Medit ...
and Iraq
Iraq,; ku, عێراق, translit=Êraq officially the Republic of Iraq, '; ku, کۆماری عێراق, translit=Komarî Êraq is a country in Western Asia. It is bordered by Turkey to the north, Iran to the east, the Persian Gulf and K ...
. The original "Israeli cohort" of patients referred to Zvi Laron and colleagues beginning in 1958 consisted of 64 patients as of 2009, including 4 deceased patients. The countries of origin of these patients include Israel, Palestine, Jordan
Jordan ( ar, الأردن; tr. ' ), officially the Hashemite Kingdom of Jordan,; tr. ' is a country in Western Asia. It is situated at the crossroads of Asia, Africa, and Europe, within the Levant region, on the East Bank of the Jordan Rive ...
, Lebanon
Lebanon ( , ar, لُبْنَان, translit=lubnān, ), officially the Republic of Lebanon () or the Lebanese Republic, is a country in Western Asia. It is located between Syria to Lebanon–Syria border, the north and east and Israel to Blue ...
, Iran
Iran, officially the Islamic Republic of Iran, and also called Persia, is a country located in Western Asia. It is bordered by Iraq and Turkey to the west, by Azerbaijan and Armenia to the northwest, by the Caspian Sea and Turkmeni ...
, Malta
Malta ( , , ), officially the Republic of Malta ( mt, Repubblika ta' Malta ), is an island country in the Mediterranean Sea. It consists of an archipelago, between Italy and Libya, and is often considered a part of Southern Europe. It lies ...
, Italy
Italy ( it, Italia ), officially the Italian Republic, ) or the Republic of Italy, is a country in Southern Europe. It is located in the middle of the Mediterranean Sea, and its territory largely coincides with the homonymous geographical ...
, Argentina
Argentina (), officially the Argentine Republic ( es, link=no, República Argentina), is a country in the southern half of South America. Argentina covers an area of , making it the second-largest country in South America after Brazil, th ...
, Ecuador
Ecuador ( ; ; Quechua: ''Ikwayur''; Shuar: ''Ecuador'' or ''Ekuatur''), officially the Republic of Ecuador ( es, República del Ecuador, which literally translates as "Republic of the Equator"; Quechua: ''Ikwadur Ripuwlika''; Shuar: ' ...
, and Peru
, image_flag = Flag of Peru.svg
, image_coat = Escudo nacional del Perú.svg
, other_symbol = Great Seal of the State
, other_symbol_type = National seal
, national_motto = "Firm and Happy f ...
.
A disproportionate number of people with the condition are found in remote villages in the Loja province of Ecuador
Ecuador ( ; ; Quechua: ''Ikwayur''; Shuar: ''Ecuador'' or ''Ekuatur''), officially the Republic of Ecuador ( es, República del Ecuador, which literally translates as "Republic of the Equator"; Quechua: ''Ikwadur Ripuwlika''; Shuar: ' ...
. These individuals are descended from colonial-era Jewish-origin New Christian
New Christian ( es, Cristiano Nuevo; pt, Cristão-Novo; ca, Cristià Nou; lad, Christiano Muevo) was a socio-religious designation and legal distinction in the Spanish Empire and the Portuguese Empire. The term was used from the 15th century ...
conversos
A ''converso'' (; ; feminine form ''conversa''), "convert", () was a Jew who converted to Catholicism in Spain or Portugal, particularly during the 14th and 15th centuries, or one of his or her descendants.
To safeguard the Old Christian p ...
(Sephardi Jews
Sephardic (or Sephardi) Jews (, ; lad, Djudíos Sefardíes), also ''Sepharadim'' , Modern Hebrew: ''Sfaradim'', Tiberian: Səp̄āraddîm, also , ''Ye'hude Sepharad'', lit. "The Jews of Spain", es, Judíos sefardíes (or ), pt, Judeus sefa ...
who themselves, or whose forebears, had been compelled to convert to Catholicism back in Spain) who had covertly migrated to Ecuador during the Spanish Conquest
The Spanish Empire ( es, link=no, Imperio español), also known as the Hispanic Monarchy ( es, link=no, Monarquía Hispánica) or the Catholic Monarchy ( es, link=no, Monarquía Católica) was a colonial empire governed by Spain and its predece ...
despite the Spanish Crown's prohibition of their emigration to its colonies and territories as a result of the Inquisition
The Inquisition was a group of institutions within the Catholic Church whose aim was to combat heresy, conducting trials of suspected heretics. Studies of the records have found that the overwhelming majority of sentences consisted of penances, ...
.
Other patients include people of other Semitic non-Jewish origins, including from Saudi Arabia, Japan, and China.
''Homo floresiensis''
Recent publications have proposed that ''Homo floresiensis
''Homo floresiensis'' also known as "Flores Man"; nicknamed "Hobbit") is an extinct species of small archaic human that inhabited the island of Flores, Indonesia, until the arrival of modern humans about 50,000 years ago.
The remains of an in ...
'' represented a population with widespread Laron syndrome, based upon the many similarities of skeletal remains found in Indonesia with LS. This is only one of several competing hypotheses, and has received criticism as insufficient to explain the "range features observed in ''H. floresiensis''". Similar postulates have been proposed regarding the Pygmies
In anthropology, pygmy peoples are ethnic groups whose average height is unusually short. The term pygmyism is used to describe the phenotype of endemic short stature (as opposed to disproportionate dwarfism occurring in isolated cases in a pop ...
of Central Africa.
History
Israelipediatric
Pediatrics ( also spelled ''paediatrics'' or ''pædiatrics'') is the branch of medicine that involves the medical care of infants, children, adolescents, and young adults. In the United Kingdom, paediatrics covers many of their youth until the ...
endocrinologist
Endocrinology (from ''endocrine'' + '' -ology'') is a branch of biology and medicine dealing with the endocrine system, its diseases, and its specific secretions known as hormones. It is also concerned with the integration of developmental events ...
Zvi Laron
Zvi Laron ( he, צבי לרון, born February 6, 1927) is an Israeli paediatric endocrinologist. Born in Cernăuţi, Romania, Laron is a professor emeritus at Tel Aviv University.
In 1966, he described the type of dwarfism later called Laron syn ...
, along with Athalia Pertzelan, Avinoam Galatzer, Liora Kornreich, Dalia Peled, Rivka Kauli, and Beatrice Klinger published the earliest clinical studies of individuals with LS beginning in 1966. Among their first 22 patients, Laron and colleagues noted consanguineous
Consanguinity ("blood relation", from Latin '' consanguinitas'') is the characteristic of having a kinship with another person (being descended from a common ancestor).
Many jurisdictions have laws prohibiting people who are related by blood fro ...
genealogy of Israeli and Palestinian ancestry with distinct physical characteristics resembling hypopituitarism
Hypopituitarism is the decreased (''hypo'') secretion of one or more of the eight hormones normally produced by the pituitary gland at the base of the brain. If there is decreased secretion of one specific pituitary hormone, the condition is know ...
. However, researchers noticed that these people had high serum GH levels, which are expected to be low in patients with hypopituitarism. Successive studies carried out over the subsequent 20 years by Laron and colleagues revealed an absence of IGF-1 release in response to exogenous hGH and an absence of GH binding to liver cell membranes in this group of patients. The results of these studies provided clear evidence that the pathogenicity of the disease was the result of GH receptor failure in the liver.
See also
* Hypothalamic–pituitary–somatic axisReferences
External links
* {{Authority control Autosomal recessive disorders Rare syndromes Syndromes affecting stature Cell surface receptor deficiencies Growth hormones Disorders causing seizures