Infrared radiation on:
[Wikipedia]
[Google]
[Amazon]
 Infrared (IR; sometimes called infrared light) is
Infrared (IR; sometimes called infrared light) is
 A third scheme divides up the band based on the response of various detectors:Miller, ''Principles of Infrared Technology'' (Van Nostrand Reinhold, 1992), and Miller and Friedman, ''Photonic Rules of Thumb'', 2004.
* Near-infrared: from 0.7 to 1.0 μm (from the approximate end of the response of the human eye to that of silicon).
* Short-wave infrared: 1.0 to 3 μm (from the cut-off of silicon to that of the MWIR atmospheric window). InGaAs covers to about 1.8 μm; the less sensitive lead salts cover this region. Cryogenically cooled MCT detectors can cover the region of 1.0–2.5μm.
* Mid-wave infrared: 3 to 5 μm (defined by the atmospheric window and covered by indium antimonide, InSb and mercury cadmium telluride, HgCdTe, and partially by lead selenide, PbSe).
* Long-wave infrared: 8 to 12, or 7 to 14 μm (this is the atmospheric window covered by HgCdTe and
A third scheme divides up the band based on the response of various detectors:Miller, ''Principles of Infrared Technology'' (Van Nostrand Reinhold, 1992), and Miller and Friedman, ''Photonic Rules of Thumb'', 2004.
* Near-infrared: from 0.7 to 1.0 μm (from the approximate end of the response of the human eye to that of silicon).
* Short-wave infrared: 1.0 to 3 μm (from the cut-off of silicon to that of the MWIR atmospheric window). InGaAs covers to about 1.8 μm; the less sensitive lead salts cover this region. Cryogenically cooled MCT detectors can cover the region of 1.0–2.5μm.
* Mid-wave infrared: 3 to 5 μm (defined by the atmospheric window and covered by indium antimonide, InSb and mercury cadmium telluride, HgCdTe, and partially by lead selenide, PbSe).
* Long-wave infrared: 8 to 12, or 7 to 14 μm (this is the atmospheric window covered by HgCdTe and
 Infrared radiation is popularly known as "heat radiation", but light and electromagnetic waves of any frequency will heat surfaces that absorb them. Infrared light from the Sun accounts for 49% of the heating of Earth, with the rest being caused by visible light that is absorbed then re-radiated at longer wavelengths. Visible light or ultraviolet-emitting lasers can char paper and incandescently hot objects emit visible radiation. Objects at room
Infrared radiation is popularly known as "heat radiation", but light and electromagnetic waves of any frequency will heat surfaces that absorb them. Infrared light from the Sun accounts for 49% of the heating of Earth, with the rest being caused by visible light that is absorbed then re-radiated at longer wavelengths. Visible light or ultraviolet-emitting lasers can char paper and incandescently hot objects emit visible radiation. Objects at room
 Infrared is used in night vision equipment when there is insufficient visible light to see. Night vision devices operate through a process involving the conversion of ambient light photons into electrons that are then amplified by a chemical and electrical process and then converted back into visible light. Infrared light sources can be used to augment the available ambient light for conversion by night vision devices, increasing in-the-dark visibility without actually using a visible light source.
The use of infrared light and night vision devices should not be confused with thermal imaging, which creates images based on differences in surface temperature by detecting infrared radiation (
Infrared is used in night vision equipment when there is insufficient visible light to see. Night vision devices operate through a process involving the conversion of ambient light photons into electrons that are then amplified by a chemical and electrical process and then converted back into visible light. Infrared light sources can be used to augment the available ambient light for conversion by night vision devices, increasing in-the-dark visibility without actually using a visible light source.
The use of infrared light and night vision devices should not be confused with thermal imaging, which creates images based on differences in surface temperature by detecting infrared radiation (
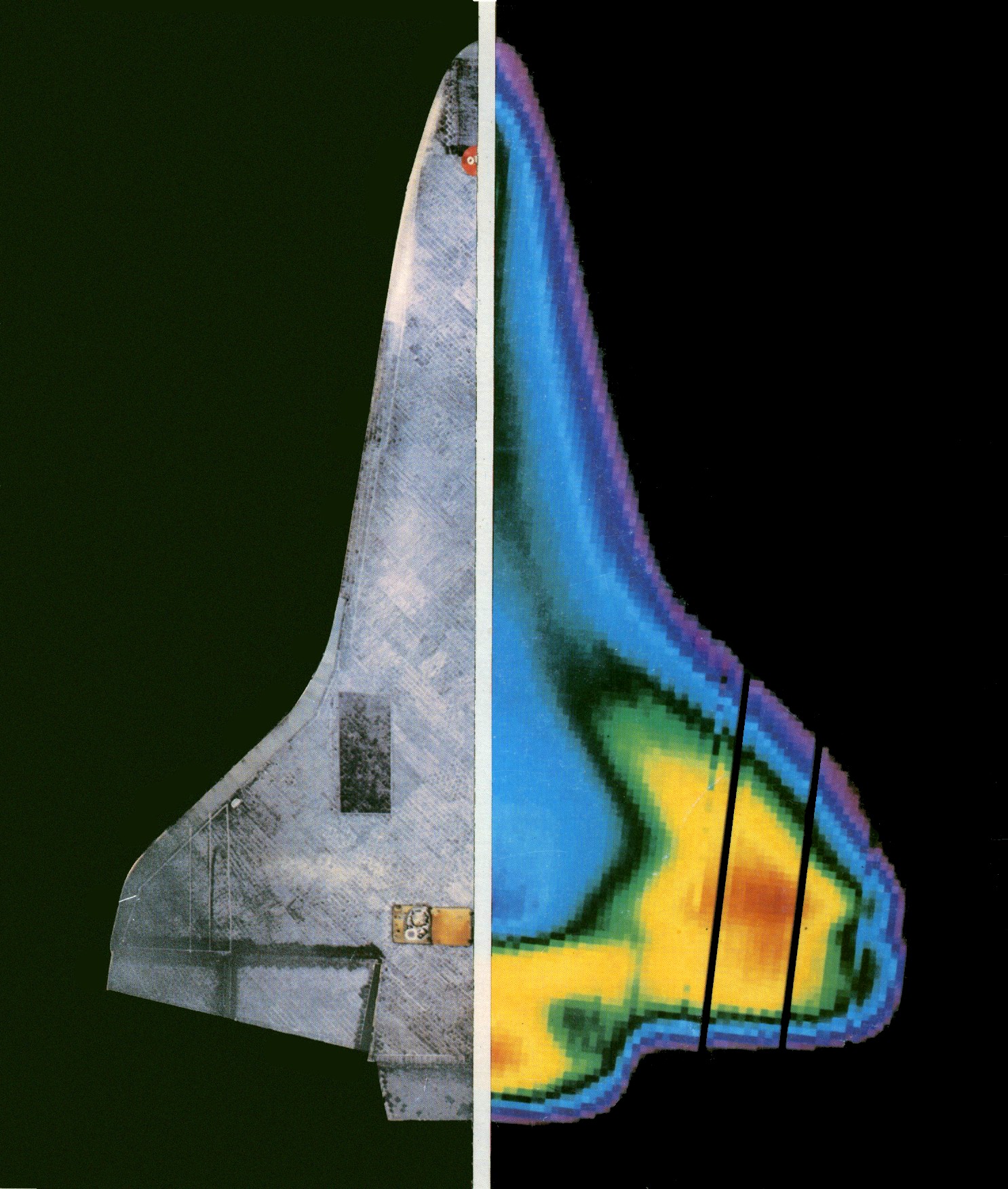 Infrared radiation can be used to remotely determine the temperature of objects (if the emissivity is known). This is termed thermography, or in the case of very hot objects in the NIR or visible it is termed pyrometry. Thermography (thermal imaging) is mainly used in military and industrial applications but the technology is reaching the public market in the form of infrared cameras on cars due to greatly reduced production costs.
Thermographic cameras detect radiation in the infrared range of the electromagnetic spectrum (roughly 9,000–14,000 nm or 9–14 μm) and produce images of that radiation. Since infrared radiation is emitted by all objects based on their temperatures, according to the black-body radiation law, thermography makes it possible to "see" one's environment with or without visible illumination. The amount of radiation emitted by an object increases with temperature, therefore thermography allows one to see variations in temperature (hence the name).
Infrared radiation can be used to remotely determine the temperature of objects (if the emissivity is known). This is termed thermography, or in the case of very hot objects in the NIR or visible it is termed pyrometry. Thermography (thermal imaging) is mainly used in military and industrial applications but the technology is reaching the public market in the form of infrared cameras on cars due to greatly reduced production costs.
Thermographic cameras detect radiation in the infrared range of the electromagnetic spectrum (roughly 9,000–14,000 nm or 9–14 μm) and produce images of that radiation. Since infrared radiation is emitted by all objects based on their temperatures, according to the black-body radiation law, thermography makes it possible to "see" one's environment with or without visible illumination. The amount of radiation emitted by an object increases with temperature, therefore thermography allows one to see variations in temperature (hence the name).
World First Thermal Hyperspectral Camera for Unmanned Aerial Vehicles
.
 In infrared photography, infrared filters are used to capture the near-infrared spectrum.
In infrared photography, infrared filters are used to capture the near-infrared spectrum.
 Infrared tracking, also known as infrared homing, refers to a passive missile guidance system, which uses the emission from a target of electromagnetic radiation in the infrared part of the spectrum to track it. Missiles that use infrared seeking are often referred to as "heat-seekers" since infrared (IR) is just below the visible spectrum of light in frequency and is radiated strongly by hot bodies. Many objects such as people, vehicle engines, and aircraft generate and retain heat, and as such, are especially visible in the infrared wavelengths of light compared to objects in the background.
Infrared tracking, also known as infrared homing, refers to a passive missile guidance system, which uses the emission from a target of electromagnetic radiation in the infrared part of the spectrum to track it. Missiles that use infrared seeking are often referred to as "heat-seekers" since infrared (IR) is just below the visible spectrum of light in frequency and is radiated strongly by hot bodies. Many objects such as people, vehicle engines, and aircraft generate and retain heat, and as such, are especially visible in the infrared wavelengths of light compared to objects in the background.
 Infrared radiation can be used as a deliberate heating source. For example, it is used in infrared saunas to heat the occupants. It may also be used in other heating applications, such as to remove ice from the wings of aircraft (de-icing).
Infrared heating is also becoming more popular in industrial manufacturing processes, e.g. curing of coatings, forming of plastics, annealing, plastic welding, and print drying. In these applications, infrared heaters replace convection ovens and contact heating.
Infrared radiation can be used as a deliberate heating source. For example, it is used in infrared saunas to heat the occupants. It may also be used in other heating applications, such as to remove ice from the wings of aircraft (de-icing).
Infrared heating is also becoming more popular in industrial manufacturing processes, e.g. curing of coatings, forming of plastics, annealing, plastic welding, and print drying. In these applications, infrared heaters replace convection ovens and contact heating.
 In the field of climatology, atmospheric infrared radiation is monitored to detect trends in the energy exchange between the Earth and the atmosphere. These trends provide information on long-term changes in Earth's climate. It is one of the primary parameters studied in research into
In the field of climatology, atmospheric infrared radiation is monitored to detect trends in the energy exchange between the Earth and the atmosphere. These trends provide information on long-term changes in Earth's climate. It is one of the primary parameters studied in research into

 Infrared reflectography can be applied to paintings to reveal underlying layers in a non-destructive manner, in particular the artist's underdrawing or outline drawn as a guide. Art conservators use the technique to examine how the visible layers of paint differ from the underdrawing or layers in between (such alterations are called pentimenti when made by the original artist). This is very useful information in deciding whether a painting is the prime version by the original artist or a copy, and whether it has been altered by over-enthusiastic restoration work. In general, the more pentimenti, the more likely a painting is to be the prime version. It also gives useful insights into working practices. Reflectography often reveals the artist's use of carbon black, which shows up well in reflectograms, as long as it has not also been used in the ground underlying the whole painting. Infrared reflectography can be realized by modified commercial digital cameras in the NIR spectral region or by dedicated instruments in the SWIR spectral region. The recent extension of reflectography into the MWIR spectral region has proved capable of detecting subtle differences in surface materials.
Finally, NIR reflectography can be performed with good results using smartphone cameras .
Recent progress in the design of infrared-sensitive cameras makes it possible to discover and depict not only underpaintings and pentimenti, but entire paintings that were later overpainted by the artist. Notable examples are Picasso's '' Woman Ironing'' and '' Blue Room'', where in both cases a portrait of a man has been made visible under the painting as it is known today.
Similar uses of infrared are made by conservators and scientists on various types of objects, especially very old written documents such as the
Infrared reflectography can be applied to paintings to reveal underlying layers in a non-destructive manner, in particular the artist's underdrawing or outline drawn as a guide. Art conservators use the technique to examine how the visible layers of paint differ from the underdrawing or layers in between (such alterations are called pentimenti when made by the original artist). This is very useful information in deciding whether a painting is the prime version by the original artist or a copy, and whether it has been altered by over-enthusiastic restoration work. In general, the more pentimenti, the more likely a painting is to be the prime version. It also gives useful insights into working practices. Reflectography often reveals the artist's use of carbon black, which shows up well in reflectograms, as long as it has not also been used in the ground underlying the whole painting. Infrared reflectography can be realized by modified commercial digital cameras in the NIR spectral region or by dedicated instruments in the SWIR spectral region. The recent extension of reflectography into the MWIR spectral region has proved capable of detecting subtle differences in surface materials.
Finally, NIR reflectography can be performed with good results using smartphone cameras .
Recent progress in the design of infrared-sensitive cameras makes it possible to discover and depict not only underpaintings and pentimenti, but entire paintings that were later overpainted by the artist. Notable examples are Picasso's '' Woman Ironing'' and '' Blue Room'', where in both cases a portrait of a man has been made visible under the painting as it is known today.
Similar uses of infrared are made by conservators and scientists on various types of objects, especially very old written documents such as the
 * 1830: Leopoldo Nobili made the first thermopile IR detector.
* 1840:
* 1830: Leopoldo Nobili made the first thermopile IR detector.
* 1840:
Infrared: A Historical Perspective
(Omega Engineering)
Infrared Data Association
, a standards organization for infrared data interconnection
detailed explanation of infrared light. (NASA)
Herschel's original paper from 1800 announcing the discovery of infrared light
The thermographic's library
, collection of thermogram
Infrared reflectography in analysis of paintings
at ColourLex * Molly Faries
Techniques and Applications – Analytical Capabilities of Infrared Reflectography: An Art Historian s Perspective
, in Scientific Examination of Art: Modern Techniques in Conservation and Analysis, Sackler NAS Colloquium, 2005 {{Authority control Electromagnetic spectrum
 Infrared (IR; sometimes called infrared light) is
Infrared (IR; sometimes called infrared light) is electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
(EMR) with wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
s longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those of red light (the longest waves in the visible spectrum
The visible spectrum is the spectral band, band of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' (or simply light).
The optica ...
), so IR is invisible to the human eye. IR is generally (according to ISO, CIE) understood to include wavelengths from around to . IR is commonly divided between longer-wavelength thermal IR, emitted from terrestrial sources, and shorter-wavelength IR or near-IR, part of the solar spectrum. Longer IR wavelengths (30–100 μm) are sometimes included as part of the terahertz radiation
Terahertz radiation – also known as submillimeter radiation, terahertz waves, tremendously high frequency
(THF), T-rays, T-waves, T-light, T-lux or THz – consists of electromagnetic waves within the International Telecommunicat ...
band. Almost all black-body radiation from objects near room temperature is in the IR band. As a form of EMR, IR carries energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium ...
and of a particle, the photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
.
It was long known that fires emit invisible heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel
Frederick William Herschel ( ; ; 15 November 1738 – 25 August 1822) was a German-British astronomer and composer. He frequently collaborated with his younger sister and fellow astronomer Caroline Herschel. Born in the Electorate of Hanover ...
discovered that infrared radiation is a type of invisible radiation in the spectrum lower in energy than red light, by means of its effect on a thermometer
A thermometer is a device that measures temperature (the hotness or coldness of an object) or temperature gradient (the rates of change of temperature in space). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb ...
.Michael Rowan-Robinson (2013). ''Night Vision: Exploring the Infrared Universe''. Cambridge University Press. p. 23. . Slightly more than half of the energy from the Sun was eventually found, through Herschel's studies, to arrive on Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
in the form of infrared. The balance between absorbed and emitted infrared radiation has an important effect on Earth's climate
Climate is the long-term weather pattern in a region, typically averaged over 30 years. More rigorously, it is the mean and variability of meteorological variables over a time spanning from months to millions of years. Some of the meteoro ...
.
Infrared radiation is emitted or absorbed by molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s when changing rotational-vibrational movements. It excites vibration
Vibration () is a mechanical phenomenon whereby oscillations occur about an equilibrium point. Vibration may be deterministic if the oscillations can be characterised precisely (e.g. the periodic motion of a pendulum), or random if the os ...
al modes in a molecule through a change in the dipole moment, making it a useful frequency range for study of these energy states for molecules of the proper symmetry. Infrared spectroscopy examines absorption and transmission of photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s in the infrared range.
Infrared radiation is used in industrial, scientific, military, commercial, and medical applications. Night-vision devices using active near-infrared illumination allow people or animals to be observed without the observer being detected. Infrared astronomy
Infrared astronomy is a sub-discipline of astronomy which specializes in the astronomical observation, observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 microm ...
uses sensor-equipped telescope
A telescope is a device used to observe distant objects by their emission, Absorption (electromagnetic radiation), absorption, or Reflection (physics), reflection of electromagnetic radiation. Originally, it was an optical instrument using len ...
s to penetrate dusty regions of space such as molecular cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...
s, to detect objects such as planet
A planet is a large, Hydrostatic equilibrium, rounded Astronomical object, astronomical body that is generally required to be in orbit around a star, stellar remnant, or brown dwarf, and is not one itself. The Solar System has eight planets b ...
s, and to view highly red-shifted objects from the early days of the universe
The universe is all of space and time and their contents. It comprises all of existence, any fundamental interaction, physical process and physical constant, and therefore all forms of matter and energy, and the structures they form, from s ...
. Infrared thermal-imaging cameras are used to detect heat loss in insulated systems, to observe changing blood flow in the skin, to assist firefighting, and to detect the overheating of electrical components. Military and civilian applications include target acquisition
Target acquisition is the detection and identification of the location of a target in sufficient detail to permit the effective employment of lethal and non-lethal means. The term is used for a broad area of applications.
A "target" here is an e ...
, surveillance
Surveillance is the monitoring of behavior, many activities, or information for the purpose of information gathering, influencing, managing, or directing. This can include observation from a distance by means of electronic equipment, such as ...
, night vision
Night vision is the ability to see in low-light conditions, either naturally with scotopic vision or through a night-vision device. Night vision requires both sufficient spectral range and sufficient intensity range. Humans have poor night v ...
, homing, and tracking. Humans at normal body temperature radiate chiefly at wavelengths around 10 μm. Non-military uses include thermal efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For ...
analysis, environmental monitoring, industrial facility inspections, detection of grow-ops, remote temperature sensing, short-range wireless communication, spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
, and weather forecasting
Weather forecasting or weather prediction is the application of science and technology forecasting, to predict the conditions of the Earth's atmosphere, atmosphere for a given location and time. People have attempted to predict the weather info ...
.
Definition and relationship to the electromagnetic spectrum
There is no universally accepted definition of the range of infrared radiation. Typically, it is taken to extend from the nominal red edge of the visible spectrum at 780 nm to 1 mm. This range of wavelengths corresponds to afrequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
range of approximately 430 THz down to 300 GHz. Beyond infrared is the microwave portion of the electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
. Increasingly, terahertz radiation is counted as part of the microwave band, not infrared, moving the band edge of infrared to 0.1 mm (3 THz).
Nature
Sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrare ...
, at an effective temperature of 5,780 K (5,510 °C, 9,940 °F), is composed of near-thermal-spectrum radiation that is slightly more than half infrared. At zenith
The zenith (, ) is the imaginary point on the celestial sphere directly "above" a particular location. "Above" means in the vertical direction (Vertical and horizontal, plumb line) opposite to the gravity direction at that location (nadir). The z ...
, sunlight provides an irradiance of just over 1 kW per square meter at sea level. Of this energy, 527 W is infrared radiation, 445 W is visible light, and 32 W is ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
radiation. Nearly all the infrared radiation in sunlight is near infrared, shorter than 4 μm.
On the surface of Earth, at far lower temperatures than the surface of the Sun, some thermal radiation consists of infrared in the mid-infrared region, much longer than in sunlight. Black-body, or thermal, radiation is continuous: it radiates at all wavelengths. Of these natural thermal radiation processes, only lightning and natural fires are hot enough to produce much visible energy, and fires produce far more infrared than visible-light energy.
Regions
In general, objects emit infrared radiation across a spectrum of wavelengths, but sometimes only a limited region of the spectrum is of interest because sensors usually collect radiation only within a specific bandwidth. Thermal infrared radiation also has a maximum emission wavelength, which is inversely proportional to the absolute temperature of object, in accordance with Wien's displacement law. The infrared band is often subdivided into smaller sections, although how the IR spectrum is thereby divided varies between different areas in which IR is employed.Visible limit
Infrared radiation is generally considered to begin with wavelengths longer than visible by the human eye. There is no hard wavelength limit to what is visible, as the eye's sensitivity decreases rapidly but smoothly, for wavelengths exceeding about 700 nm. Therefore wavelengths just longer than that can be seen if they are sufficiently bright, though they may still be classified as infrared according to usual definitions. Light from a near-IR laser may thus appear dim red and can present a hazard since it may actually carry a large amount of energy. Even IR at wavelengths up to 1,050 nm from pulsed lasers can be seen by humans under certain conditions.Commonly used subdivision scheme
A commonly used subdivision scheme is: NIR and SWIR together is sometimes called "reflected infrared", whereas MWIR and LWIR is sometimes referred to as "thermal infrared".CIE division scheme
The International Commission on Illumination (CIE) recommended the division of infrared radiation into the following three bands:ISO 20473 scheme
ISO
The International Organization for Standardization (ISO ; ; ) is an independent, non-governmental, international standard development organization composed of representatives from the national standards organizations of member countries.
Me ...
20473 specifies the following scheme:
Astronomy division scheme
Astronomers typically divide the infrared spectrum as follows: These divisions are not precise and can vary depending on the publication. The three regions are used for observation of different temperature ranges, and hence different environments in space. The most common photometric system used in astronomy allocates capital letters to different spectral regions according to filters used; I, J, H, and K cover the near-infrared wavelengths; L, M, N, and Q refer to the mid-infrared region. These letters are commonly understood in reference to atmospheric windows and appear, for instance, in the titles of many papers.Sensor response division scheme
microbolometer
A microbolometer is a specific type of bolometer used as a detector in a thermal camera. Infrared radiation with wavelengths between 7.5–14 μm strikes the detector material, heating it, and thus changing its electrical resistance. This resista ...
s).
* Very-long wave infrared (VLWIR) (12 to about 30 μm, covered by doped silicon).
Near-infrared is the region closest in wavelength to the radiation detectable by the human eye. mid- and far-infrared are progressively further from the visible spectrum. Other definitions follow different physical mechanisms (emission peaks, vs. bands, water absorption) and the newest follow technical reasons (the common silicon detectors are sensitive to about 1,050 nm, while InGaAs's sensitivity starts around 950 nm and ends between 1,700 and 2,600 nm, depending on the specific configuration). No international standards for these specifications are currently available.
The onset of infrared is defined (according to different standards) at various values typically between 700 nm and 800 nm, but the boundary between visible and infrared light is not precisely defined. The human eye is markedly less sensitive to light above 700 nm wavelength, so longer wavelengths make insignificant contributions to scenes illuminated by common light sources. Particularly intense near-IR light (e.g., from laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s, LEDs or bright daylight with the visible light filtered out) can be detected up to approximately 780 nm, and will be perceived as red light. Intense light sources providing wavelengths as long as 1,050 nm can be seen as a dull red glow, causing some difficulty in near-IR illumination of scenes in the dark (usually this practical problem is solved by indirect illumination). Leaves are particularly bright in the near IR, and if all visible light leaks from around an IR-filter are blocked, and the eye is given a moment to adjust to the extremely dim image coming through a visually opaque IR-passing photographic filter, it is possible to see the Wood effect that consists of IR-glowing foliage.
Telecommunication bands
In optical communications, the part of the infrared spectrum that is used is divided into seven bands based on availability of light sources, transmitting/absorbing materials (fibers), and detectors: The C-band is the dominant band for long-distancetelecommunications network
A telecommunications network is a group of Node (networking), nodes interconnected by telecommunications links that are used to exchange messages between the nodes. The links may use a variety of technologies based on the methodologies of circuit ...
s. The S and L bands are based on less well established technology, and are not as widely deployed.
Heat
 Infrared radiation is popularly known as "heat radiation", but light and electromagnetic waves of any frequency will heat surfaces that absorb them. Infrared light from the Sun accounts for 49% of the heating of Earth, with the rest being caused by visible light that is absorbed then re-radiated at longer wavelengths. Visible light or ultraviolet-emitting lasers can char paper and incandescently hot objects emit visible radiation. Objects at room
Infrared radiation is popularly known as "heat radiation", but light and electromagnetic waves of any frequency will heat surfaces that absorb them. Infrared light from the Sun accounts for 49% of the heating of Earth, with the rest being caused by visible light that is absorbed then re-radiated at longer wavelengths. Visible light or ultraviolet-emitting lasers can char paper and incandescently hot objects emit visible radiation. Objects at room temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
will emit radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
* ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infr ...
concentrated mostly in the 8 to 25 μm band, but this is not distinct from the emission of visible light by incandescent objects and ultraviolet by even hotter objects (see black body and Wien's displacement law).
Heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
is energy in transit that flows due to a temperature difference. Unlike heat transmitted by thermal conduction
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy ...
or thermal convection, thermal radiation can propagate through a vacuum. Thermal radiation is characterized by a particular spectrum of many wavelengths that are associated with emission from an object, due to the vibration of its molecules at a given temperature. Thermal radiation can be emitted from objects at any wavelength, and at very high temperatures such radiation is associated with spectra far above the infrared, extending into visible, ultraviolet, and even X-ray regions (e.g. the solar corona
In astronomy, a corona (: coronas or coronae) is the outermost layer of a star's Stellar atmosphere, atmosphere. It is a hot but relatively luminosity, dim region of Plasma (physics), plasma populated by intermittent coronal structures such as so ...
). Thus, the popular association of infrared radiation with thermal radiation is only a coincidence based on typical (comparatively low) temperatures often found near the surface of planet Earth.
The concept of emissivity is important in understanding the infrared emissions of objects. This is a property of a surface that describes how its thermal emissions deviate from the ideal of a black body. To further explain, two objects at the same physical temperature may not show the same infrared image if they have differing emissivity. For example, for any pre-set emissivity value, objects with higher emissivity will appear hotter, and those with a lower emissivity will appear cooler (assuming, as is often the case, that the surrounding environment is cooler than the objects being viewed). When an object has less than perfect emissivity, it obtains properties of reflectivity and/or transparency, and so the temperature of the surrounding environment is partially reflected by and/or transmitted through the object. If the object were in a hotter environment, then a lower emissivity object at the same temperature would likely appear to be hotter than a more emissive one. For that reason, incorrect selection of emissivity and not accounting for environmental temperatures will give inaccurate results when using infrared cameras and pyrometers.
Applications
Night vision
 Infrared is used in night vision equipment when there is insufficient visible light to see. Night vision devices operate through a process involving the conversion of ambient light photons into electrons that are then amplified by a chemical and electrical process and then converted back into visible light. Infrared light sources can be used to augment the available ambient light for conversion by night vision devices, increasing in-the-dark visibility without actually using a visible light source.
The use of infrared light and night vision devices should not be confused with thermal imaging, which creates images based on differences in surface temperature by detecting infrared radiation (
Infrared is used in night vision equipment when there is insufficient visible light to see. Night vision devices operate through a process involving the conversion of ambient light photons into electrons that are then amplified by a chemical and electrical process and then converted back into visible light. Infrared light sources can be used to augment the available ambient light for conversion by night vision devices, increasing in-the-dark visibility without actually using a visible light source.
The use of infrared light and night vision devices should not be confused with thermal imaging, which creates images based on differences in surface temperature by detecting infrared radiation (heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
) that emanates from objects and their surrounding environment.
Thermography
 Infrared radiation can be used to remotely determine the temperature of objects (if the emissivity is known). This is termed thermography, or in the case of very hot objects in the NIR or visible it is termed pyrometry. Thermography (thermal imaging) is mainly used in military and industrial applications but the technology is reaching the public market in the form of infrared cameras on cars due to greatly reduced production costs.
Thermographic cameras detect radiation in the infrared range of the electromagnetic spectrum (roughly 9,000–14,000 nm or 9–14 μm) and produce images of that radiation. Since infrared radiation is emitted by all objects based on their temperatures, according to the black-body radiation law, thermography makes it possible to "see" one's environment with or without visible illumination. The amount of radiation emitted by an object increases with temperature, therefore thermography allows one to see variations in temperature (hence the name).
Infrared radiation can be used to remotely determine the temperature of objects (if the emissivity is known). This is termed thermography, or in the case of very hot objects in the NIR or visible it is termed pyrometry. Thermography (thermal imaging) is mainly used in military and industrial applications but the technology is reaching the public market in the form of infrared cameras on cars due to greatly reduced production costs.
Thermographic cameras detect radiation in the infrared range of the electromagnetic spectrum (roughly 9,000–14,000 nm or 9–14 μm) and produce images of that radiation. Since infrared radiation is emitted by all objects based on their temperatures, according to the black-body radiation law, thermography makes it possible to "see" one's environment with or without visible illumination. The amount of radiation emitted by an object increases with temperature, therefore thermography allows one to see variations in temperature (hence the name).
Hyperspectral imaging
A hyperspectral image is a "picture" containing continuousspectrum
A spectrum (: spectra or spectrums) is a set of related ideas, objects, or properties whose features overlap such that they blend to form a continuum. The word ''spectrum'' was first used scientifically in optics to describe the rainbow of co ...
through a wide spectral range at each pixel. Hyperspectral imaging is gaining importance in the field of applied spectroscopy particularly with NIR, SWIR, MWIR, and LWIR spectral regions. Typical applications include biological, mineralogical, defence, and industrial measurements.
Thermal infrared hyperspectral imaging can be similarly performed using a thermographic camera
Infrared thermography (IRT), thermal video or thermal imaging, is a process where a Thermographic camera, thermal camera captures and creates an image of an object by using infrared radiation emitted from the object in a process, which are exa ...
, with the fundamental difference that each pixel contains a full LWIR spectrum. Consequently, chemical identification of the object can be performed without a need for an external light source such as the Sun or the Moon. Such cameras are typically applied for geological measurements, outdoor surveillance and UAV applications.Frost&Sullivan, Technical Insights, Aerospace&Defence (Feb 2011)World First Thermal Hyperspectral Camera for Unmanned Aerial Vehicles
.
Other imaging
 In infrared photography, infrared filters are used to capture the near-infrared spectrum.
In infrared photography, infrared filters are used to capture the near-infrared spectrum. Digital camera
A digital camera, also called a digicam, is a camera that captures photographs in Digital data storage, digital memory. Most cameras produced today are digital, largely replacing those that capture images on photographic film or film stock. Dig ...
s often use infrared blockers. Cheaper digital cameras and camera phones have less effective filters and can view intense near-infrared, appearing as a bright purple-white color. This is especially pronounced when taking pictures of subjects near IR-bright areas (such as near a lamp), where the resulting infrared interference can wash out the image. There is also a technique called ' T-ray' imaging, which is imaging using far-infrared or terahertz radiation
Terahertz radiation – also known as submillimeter radiation, terahertz waves, tremendously high frequency
(THF), T-rays, T-waves, T-light, T-lux or THz – consists of electromagnetic waves within the International Telecommunicat ...
. Lack of bright sources can make terahertz photography more challenging than most other infrared imaging techniques. Recently T-ray imaging has been of considerable interest due to a number of new developments such as terahertz time-domain spectroscopy.
Tracking
Heating
 Infrared radiation can be used as a deliberate heating source. For example, it is used in infrared saunas to heat the occupants. It may also be used in other heating applications, such as to remove ice from the wings of aircraft (de-icing).
Infrared heating is also becoming more popular in industrial manufacturing processes, e.g. curing of coatings, forming of plastics, annealing, plastic welding, and print drying. In these applications, infrared heaters replace convection ovens and contact heating.
Infrared radiation can be used as a deliberate heating source. For example, it is used in infrared saunas to heat the occupants. It may also be used in other heating applications, such as to remove ice from the wings of aircraft (de-icing).
Infrared heating is also becoming more popular in industrial manufacturing processes, e.g. curing of coatings, forming of plastics, annealing, plastic welding, and print drying. In these applications, infrared heaters replace convection ovens and contact heating.
Cooling
A variety of technologies or proposed technologies take advantage of infrared emissions to cool buildings or other systems. The LWIR (8–15 μm) region is especially useful since some radiation at these wavelengths can escape into space through the atmosphere's infrared window. This is howpassive daytime radiative cooling
Passive daytime radiative cooling (PDRC) (also passive radiative cooling, daytime passive radiative cooling, radiative sky cooling, photonic radiative cooling, and terrestrial radiative cooling) is the use of unpowered, reflective/Emissivity, ther ...
(PDRC) surfaces are able to achieve sub-ambient cooling temperatures under direct solar intensity, enhancing terrestrial heat flow to outer space with zero energy consumption
Energy consumption is the amount of energy used.
Biology
In the body, energy consumption is part of energy homeostasis. It derived from food energy. Energy consumption in the body is a product of the basal metabolic rate and the physical acti ...
or pollution
Pollution is the introduction of contaminants into the natural environment that cause harm. Pollution can take the form of any substance (solid, liquid, or gas) or energy (such as radioactivity, heat, sound, or light). Pollutants, the component ...
. PDRC surfaces maximize shortwave solar reflectance to lessen heat gain while maintaining strong longwave infrared (LWIR) thermal radiation heat transfer
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, ...
. When imagined on a worldwide scale, this cooling method has been proposed as a way to slow and even reverse global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
, with some estimates proposing a global surface area coverage of 1-2% to balance global heat fluxes.
Communications
IR data transmission is also employed in short-range communication among computer peripherals andpersonal digital assistant
A personal digital assistant (PDA) is a multi-purpose mobile device which functions as a personal information manager. Following a boom in the 1990s and 2000s, PDAs were mostly displaced by the widespread adoption of more highly capable smar ...
s. These devices usually conform to standards published by IrDA, the Infrared Data Association. Remote controls and IrDA devices use infrared light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corre ...
s (LEDs) to emit infrared radiation that may be concentrated by a lens
A lens is a transmissive optical device that focuses or disperses a light beam by means of refraction. A simple lens consists of a single piece of transparent material, while a compound lens consists of several simple lenses (''elements'') ...
into a beam that the user aims at the detector. The beam is modulated, i.e. switched on and off, according to a code which the receiver interprets. Usually very near-IR is used (below 800 nm) for practical reasons. This wavelength is efficiently detected by inexpensive silicon photodiode
A photodiode is a semiconductor diode sensitive to photon radiation, such as visible light, infrared or ultraviolet radiation, X-rays and gamma rays. It produces an electrical current when it absorbs photons. This can be used for detection and me ...
s, which the receiver uses to convert the detected radiation to an electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
. That electrical signal is passed through a high-pass filter which retains the rapid pulsations due to the IR transmitter but filters out slowly changing infrared radiation from ambient light. Infrared communications are useful for indoor use in areas of high population density. IR does not penetrate walls and so does not interfere with other devices in adjoining rooms. Infrared is the most common way for remote control
A remote control, also known colloquially as a remote or clicker, is an consumer electronics, electronic device used to operate another device from a distance, usually wirelessly. In consumer electronics, a remote control can be used to operat ...
s to command appliances.
Infrared remote control protocols like RC-5, SIRC, are used to communicate with infrared.
Free-space optical communication using infrared laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s can be a relatively inexpensive way to install a communications link in an urban area operating at up to 4 gigabit/s, compared to the cost of burying fiber optic cable, except for the radiation damage. "Since the eye cannot detect IR, blinking or closing the eyes to help prevent or reduce damage may not happen."
Infrared lasers are used to provide the light for optical fiber communications systems. Wavelengths around 1,330 nm (least dispersion) or 1,550 nm (best transmission) are the best choices for standard silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
fibers.
IR data transmission of audio versions of printed signs is being researched as an aid for visually impaired people through the Remote infrared audible signage project.
Transmitting IR data from one device to another is sometimes referred to as beaming.
IR is sometimes used for assistive audio as an alternative to an audio induction loop.
Spectroscopy
Infrared vibrational spectroscopy (see also near-infrared spectroscopy) is a technique that can be used to identify molecules by analysis of their constituent bonds. Each chemical bond in a molecule vibrates at a frequency characteristic of that bond. A group of atoms in a molecule (e.g., CH2) may have multiple modes of oscillation caused by the stretching and bending motions of the group as a whole. If an oscillation leads to a change in dipole in the molecule then it will absorb aphoton
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
that has the same frequency. The vibrational frequencies of most molecules correspond to the frequencies of infrared light. Typically, the technique is used to study organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s using light radiation from the mid-infrared, 4,000–400 cm−1. A spectrum of all the frequencies of absorption in a sample is recorded. This can be used to gain information about the sample composition in terms of chemical groups present and also its purity (for example, a wet sample will show a broad O-H absorption around 3200 cm−1). The unit for expressing radiation in this application, cm−1, is the spectroscopic wavenumber. It is the frequency divided by the speed of light in vacuum.
Thin film metrology
In the semiconductor industry, infrared light can be used to characterize materials such as thin films and periodic trench structures. By measuring the reflectance of light from the surface of a semiconductor wafer, the index of refraction (n) and the extinction Coefficient (k) can be determined via the Forouhi–Bloomer dispersion equations. The reflectance from the infrared light can also be used to determine the critical dimension, depth, and sidewall angle of high aspect ratio trench structures.Meteorology
Weather satellites equipped with scanning radiometers produce thermal or infrared images, which can then enable a trained analyst to determine cloud heights and types, to calculate land and surface water temperatures, and to locate ocean surface features. The scanning is typically in the range 10.3–12.5 μm (IR4 and IR5 channels). Clouds with high and cold tops, such as cyclones orcumulonimbus cloud
Cumulonimbus () is a dense, towering, vertical cloud, typically forming from water vapor condensing in the lower troposphere that builds upward carried by powerful buoyant air currents. Above the lower portions of the cumulonimbus the water ...
s, are often displayed as red or black, lower warmer clouds such as stratus or stratocumulus are displayed as blue or grey, with intermediate clouds shaded accordingly. Hot land surfaces are shown as dark-grey or black. One disadvantage of infrared imagery is that low clouds such as stratus or fog can have a temperature similar to the surrounding land or sea surface and do not show up. However, using the difference in brightness of the IR4 channel (10.3–11.5 μm) and the near-infrared channel (1.58–1.64 μm), low clouds can be distinguished, producing a ''fog'' satellite picture. The main advantage of infrared is that images can be produced at night, allowing a continuous sequence of weather to be studied.
These infrared pictures can depict ocean eddies or vortices and map currents such as the Gulf Stream, which are valuable to the shipping industry. Fishermen and farmers are interested in knowing land and water temperatures to protect their crops against frost or increase their catch from the sea. Even El Niño phenomena can be spotted. Using color-digitized techniques, the gray-shaded thermal images can be converted to color for easier identification of desired information.
The main water vapour channel at 6.40 to 7.08 μm can be imaged by some weather satellites and shows the amount of moisture in the atmosphere.
Climatology
global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
, together with solar radiation
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible light perceptible to the human eye as well as invisible infrared (typically p ...
.
A pyrgeometer is utilized in this field of research to perform continuous outdoor measurements. This is a broadband infrared radiometer with sensitivity for infrared radiation between approximately 4.5 μm and 50 μm.
Astronomy
Astronomers observe objects in the infrared portion of the electromagnetic spectrum using optical components, including mirrors, lenses and solid state digital detectors. For this reason it is classified as part of optical astronomy. To form an image, the components of an infrared telescope need to be carefully shielded from heat sources, and the detectors are chilled using liquidhelium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
.
The sensitivity of Earth-based infrared telescopes is significantly limited by water vapor in the atmosphere, which absorbs a portion of the infrared radiation arriving from space outside of selected atmospheric windows. This limitation can be partially alleviated by placing the telescope observatory at a high altitude, or by carrying the telescope aloft with a balloon or an aircraft. Space telescopes do not suffer from this handicap, and so outer space is considered the ideal location for infrared astronomy.
The infrared portion of the spectrum has several useful benefits for astronomers. Cold, dark molecular cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...
s of gas and dust in our galaxy will glow with radiated heat as they are irradiated by imbedded stars. Infrared can also be used to detect protostar
A protostar is a very young star that is still gathering mass from its parent molecular cloud. It is the earliest phase in the process of stellar evolution. For a low-mass star (i.e. that of the Sun or lower), it lasts about 500,000 years. The p ...
s before they begin to emit visible light. Stars emit a smaller portion of their energy in the infrared spectrum, so nearby cool objects such as planet
A planet is a large, Hydrostatic equilibrium, rounded Astronomical object, astronomical body that is generally required to be in orbit around a star, stellar remnant, or brown dwarf, and is not one itself. The Solar System has eight planets b ...
s can be more readily detected. (In the visible light spectrum, the glare from the star will drown out the reflected light from a planet.)
Infrared light is also useful for observing the cores of active galaxies, which are often cloaked in gas and dust. Distant galaxies with a high redshift
In physics, a redshift is an increase in the wavelength, and corresponding decrease in the frequency and photon energy, of electromagnetic radiation (such as light). The opposite change, a decrease in wavelength and increase in frequency and e ...
will have the peak portion of their spectrum shifted toward longer wavelengths, so they are more readily observed in the infrared.
Cleaning
Infrared cleaning is a technique used by some motion picture film scanners, film scanners and flatbed scanners to reduce or remove the effect of dust and scratches upon the finished scan. It works by collecting an additional infrared channel from the scan at the same position and resolution as the three visible color channels (red, green, and blue). The infrared channel, in combination with the other channels, is used to detect the location of scratches and dust. Once located, those defects can be corrected by scaling or replaced by inpainting.Art conservation and analysis

Dead Sea Scrolls
The Dead Sea Scrolls, also called the Qumran Caves Scrolls, are a set of List of Hebrew Bible manuscripts, ancient Jewish manuscripts from the Second Temple period (516 BCE – 70 CE). They were discovered over a period of ten years, between ...
, the Roman works in the Villa of the Papyri
The Villa of the Papyri (, also known as ''Villa dei Pisoni'' and in early excavation records as the ''Villa Suburbana'') was an ancient Roman Empire, Roman villa in Herculaneum, in what is now Ercolano, southern Italy. It is named after its un ...
, and the Silk Road texts found in the Dunhuang Caves. Carbon black used in ink can show up extremely well.
Biological systems
The pit viper has a pair of infrared sensory pits on its head. There is uncertainty regarding the exact thermal sensitivity of this biological infrared detection system. Other organisms that have thermoreceptive organs are pythons (family Pythonidae), some boas (family Boidae), the Common Vampire Bat (''Desmodus rotundus''), a variety of jewel beetles ('' Melanophila acuminata''), darkly pigmented butterflies ('' Pachliopta aristolochiae'' and '' Troides rhadamantus plateni''), and possibly blood-sucking bugs ('' Triatoma infestans''). By detecting the heat that their prey emits, crotaline and boid snakes identify and capture their prey using their IR-sensitive pit organs. Comparably, IR-sensitive pits on the Common Vampire Bat (''Desmodus rotundus'') aid in the identification of blood-rich regions on its warm-blooded victim. The jewel beetle, '' Melanophila acuminata'', locatesforest fires A forest fire
A wildfire, forest fire, or a bushfire is an unplanned and uncontrolled fire in an area of combustible vegetation. Depending on the type of vegetation present, a wildfire may be more specifically identified as a bushfire ( in Au ...
via infrared pit organs, where on recently burnt trees, they deposit their eggs. Thermoreceptors on the wings and antennae of butterflies with dark pigmentation, such '' Pachliopta aristolochiae'' and '' Troides rhadamantus plateni'', shield them from heat damage as they sunbathe in the sun. Additionally, it's hypothesised that thermoreceptors let bloodsucking bugs ('' Triatoma infestans'') locate their warm-blooded victims by sensing their body heat.
Some fungi like ''Venturia inaequalis
''Venturia inaequalis'' is an ascomycota, ascomycete fungus that causes the apple scab disease.
Systematics
''Venturia inaequalis'' anamorphs have been described under the names ''Fusicladium dendriticum'' and ''Spilocaea pomi''. Whether ''V. in ...
'' require near-infrared light for ejection.
Although near-infrared vision (780–1,000 nm) has long been deemed impossible due to noise in visual pigments, sensation of near-infrared light was reported in the common carp and in three cichlid species. Fish use NIR to capture prey and for phototactic swimming orientation. NIR sensation in fish may be relevant under poor lighting conditions during twilight and in turbid surface waters.
Photobiomodulation
Near-infrared light, orphotobiomodulation
Low-level laser therapy (LLLT), cold laser therapy or photobiomodulation (PBM) is a medical treatment approach that applies low-level (low- power) lasers or light-emitting diodes (LEDs) to the surface of the body. Whereas high-power lasers are ...
, is used for treatment of chemotherapy-induced oral ulceration as well as wound healing. There is some work relating to anti-herpes virus treatment. Research projects include work on central nervous system healing effects via cytochrome c oxidase upregulation and other possible mechanisms.
Health hazards
Strong infrared radiation in certain industry high-heat settings may be hazardous to the eyes, resulting in damage or blindness to the user. Since the radiation is invisible, special IR-proof goggles must be worn in such places.Scientific history
The discovery of infrared radiation is ascribed toWilliam Herschel
Frederick William Herschel ( ; ; 15 November 1738 – 25 August 1822) was a German-British astronomer and composer. He frequently collaborated with his younger sister and fellow astronomer Caroline Herschel. Born in the Electorate of Hanover ...
, the astronomer
An astronomer is a scientist in the field of astronomy who focuses on a specific question or field outside the scope of Earth. Astronomers observe astronomical objects, such as stars, planets, natural satellite, moons, comets and galaxy, galax ...
, in the early 19th century. Herschel published his results in 1800 before the Royal Society of London. Herschel used a prism to refract light from the sun and detected the infrared, beyond the red part of the spectrum, through an increase in the temperature recorded on a thermometer
A thermometer is a device that measures temperature (the hotness or coldness of an object) or temperature gradient (the rates of change of temperature in space). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb ...
. He was surprised at the result and called them "Calorific Rays". The term "infrared" did not appear until late 19th century. The Latin prefix ''infra-'' means below, as it is light below red on the spectrum. An earlier experiment in 1790 by Marc-Auguste Pictet demonstrated the reflection and focusing of radiant heat via mirrors in the absence of visible light.
Other important dates include:
 * 1830: Leopoldo Nobili made the first thermopile IR detector.
* 1840:
* 1830: Leopoldo Nobili made the first thermopile IR detector.
* 1840: John Herschel
Sir John Frederick William Herschel, 1st Baronet (; 7 March 1792 – 11 May 1871) was an English polymath active as a mathematician, astronomer, chemist, inventor and experimental photographer who invented the blueprint and did botanical work. ...
produces the first thermal image, called a thermogram.
* 1860: Gustav Kirchhoff formulated the blackbody theorem .
* 1873: Willoughby Smith discovered the photoconductivity of selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
.
* 1878: Samuel Pierpont Langley invents the first bolometer, a device which is able to measure small temperature fluctuations, and thus the power of far infrared sources.
* 1879: Stefan–Boltzmann law formulated empirically that the power radiated by a blackbody is proportional to ''T''4.
* 1880s and 1890s: Lord Rayleigh and Wilhelm Wien solved part of the blackbody equation, but both solutions diverged in parts of the electromagnetic spectrum. This problem was called the " ultraviolet catastrophe and infrared catastrophe".
* 1892: Willem Henri Julius published infrared spectra of 20 organic compounds measured with a bolometer in units of angular displacement.
* 1901: Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
published the blackbody equation and theorem. He solved the problem by quantizing the allowable energy transitions.
* 1905: Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
developed the theory of the photoelectric effect.
* 1905–1908: William Coblentz published infrared spectra in units of wavelength (micrometers) for several chemical compounds in ''Investigations of Infra-Red Spectra''.
* 1917: Theodore Case developed the thallous sulfide detector, which helped produce the first infrared search and track device able to detect aircraft at a range of one mile (1.6 km).
* 1935: Lead salts – early missile guidance in World War II
World War II or the Second World War (1 September 1939 – 2 September 1945) was a World war, global conflict between two coalitions: the Allies of World War II, Allies and the Axis powers. World War II by country, Nearly all of the wo ...
.
* 1938: Yeou Ta predicted that the pyroelectric effect could be used to detect infrared radiation.
* 1945: The Zielgerät 1229 "Vampir" infrared weapon system was introduced as the first portable infrared device for military applications.
* 1952: Heinrich Welker
Heinrich Johann Welker (9 September 1912 in Ingolstadt – 25 December 1981 in Erlangen) was a German theoretical and applied physicist who invented the " transistron", a transistor made at Westinghouse independently of the first successful transi ...
grew synthetic InSb crystals.
* 1950s and 1960s: Nomenclature and radiometric units defined by Fred Nicodemenus, G. J. Zissis and R. Clark; Robert Clark Jones defined ''D''*.
* 1958: W. D. Lawson ( Royal Radar Establishment in Malvern) discovered IR detection properties of Mercury cadmium telluride (HgCdTe).
* 1958: Falcon and Sidewinder missiles were developed using infrared technology.
* 1960s: Paul Kruse and his colleagues at Honeywell Research Center demonstrate the use of HgCdTe as an effective compound for infrared detection.
* 1962: J. Cooper demonstrated pyroelectric detection.
* 1964: W. G. Evans discovered infrared thermoreceptors in a pyrophile beetle.
* 1965: First IR handbook; first commercial imagers ( Barnes, Agema (now part of FLIR Systems Inc.)); Richard Hudson's landmark text; F4 TRAM FLIR by Hughes; phenomenology pioneered by Fred Simmons and A. T. Stair; U.S. Army's night vision lab formed (now Night Vision and Electronic Sensors Directorate (NVESD)), and Rachets develops detection, recognition and identification modeling there.
* 1970: Willard Boyle and George E. Smith proposed CCD at Bell Labs
Nokia Bell Labs, commonly referred to as ''Bell Labs'', is an American industrial research and development company owned by Finnish technology company Nokia. With headquarters located in Murray Hill, New Jersey, Murray Hill, New Jersey, the compa ...
for picture phone.
* 1973: Common module program started by NVESD.
* 1978: Infrared imaging astronomy came of age, observatories planned, IRTF on Mauna Kea opened; 32 × 32 and 64 × 64 arrays produced using InSb, HgCdTe and other materials.
* 2013: On 14 February, researchers developed a neural implant that gives rats the ability to sense infrared light, which for the first time provides living creatures with new abilities, instead of simply replacing or augmenting existing abilities.
See also
Notes
References
External links
Infrared: A Historical Perspective
(Omega Engineering)
Infrared Data Association
, a standards organization for infrared data interconnection
detailed explanation of infrared light. (NASA)
Herschel's original paper from 1800 announcing the discovery of infrared light
The thermographic's library
, collection of thermogram
Infrared reflectography in analysis of paintings
at ColourLex * Molly Faries
Techniques and Applications – Analytical Capabilities of Infrared Reflectography: An Art Historian s Perspective
, in Scientific Examination of Art: Modern Techniques in Conservation and Analysis, Sackler NAS Colloquium, 2005 {{Authority control Electromagnetic spectrum