Industrial enzymes on:
[Wikipedia]
[Google]
[Amazon]
Industrial enzymes are
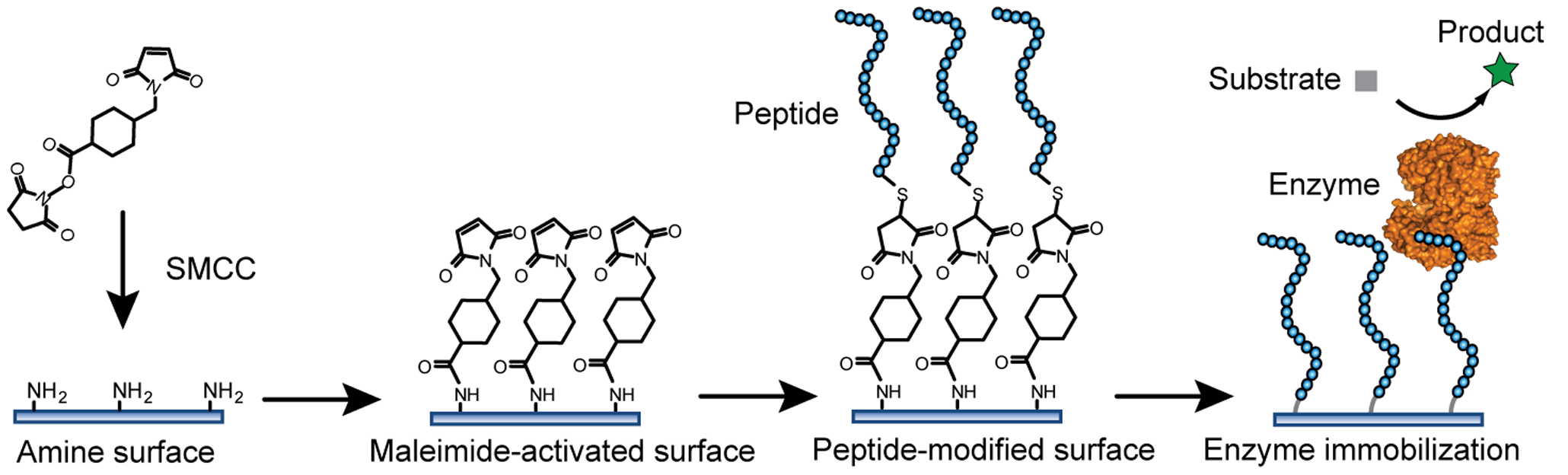 Many binding chemistries may be used to adhere an enzyme to a surface to varying degrees of success. The most successful covalent binding techniques include binding via
Many binding chemistries may be used to adhere an enzyme to a surface to varying degrees of success. The most successful covalent binding techniques include binding via
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s that are commercially used in a variety of industries such as pharmaceuticals, chemical production, biofuels, food & beverage, and consumer products. Due to advancements in recent years, biocatalysis through isolated enzymes is considered more economical than use of whole cells. Enzymes may be used as a unit operation
In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomeriza ...
within a process to generate a desired product, or may be the product of interest. Industrial biological catalysis through enzymes has experienced rapid growth in recent years due to their ability to operate at mild conditions, and exceptional chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
and positional specificity, things that traditional chemical processes lack. Isolated enzymes are typically used in hydrolytic
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
and isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
reactions. Whole cells are typically used when a reaction requires a co-factor. Although co-factors may be generated in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology ...
, it is typically more cost-effective to use metabolically active cells.
Enzymes as a unit of operation
Immobilization
Despite their excellent catalytic capabilities, enzymes and their properties must be improved prior to industrial implementation in many cases. Some aspects of enzymes that must be improved prior to implementation are stability, activity, inhibition by reaction products, and selectivity towards non-natural substrates. This may be accomplished through immobilization of enzymes on a solid material, such as a porous support. Immobilization of enzymes greatly simplifies the recovery process, enhances process control, and reduces operational costs. Many immobilization techniques exist, such as adsorption, covalent binding, affinity, and entrapment. Ideal immobilization processes should not use highly toxic reagents in the immobilization technique to ensure stability of the enzymes. After immobilization is complete, the enzymes are introduced into a reaction vessel for biocatalysis.Adsorption
Enzymeadsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
onto carriers functions based on chemical and physical phenomena such as van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s, ionic interactions, and hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
. These forces are weak, and as a result, do not affect the structure of the enzyme. A wide variety of enzyme carriers may be used. Selection of a carrier is dependent upon the surface area, particle size, pore structure, and type of functional group.
Covalent binding
 Many binding chemistries may be used to adhere an enzyme to a surface to varying degrees of success. The most successful covalent binding techniques include binding via
Many binding chemistries may be used to adhere an enzyme to a surface to varying degrees of success. The most successful covalent binding techniques include binding via glutaraldehyde
Glutaraldehyde is an organic compound with the formula . The molecule consists of a five carbon chain doubly terminated with formyl (CHO) groups. It is usually used as a solution in water, and such solutions exists as a collection of hydrates, c ...
to amino group
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
s and N-hydroxysuccinide ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s. These immobilization techniques occur at ambient temperatures in mild conditions, which have limited potential to modify the structure and function of the enzyme.
Affinity
Immobilization usingaffinity
Affinity may refer to:
Commerce, finance and law
* Affinity (law), kinship by marriage
* Affinity analysis, a market research and business management technique
* Affinity Credit Union, a Saskatchewan-based credit union
* Affinity Equity Par ...
relies on the specificity of an enzyme to couple an affinity ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
to an enzyme to form a covalently bound enzyme-ligand complex. The complex is introduced into a support matrix for which the ligand has high binding affinity, and the enzyme is immobilized through ligand-support interactions.
Entrapment
Immobilization using entrapment relies on trapping enzymes within gels or fibers, using non-covalent interactions. Characteristics that define a successful entrapping material include high surface area, uniform pore distribution, tunable pore size, and high adsorption capacity.Recovery
Enzymes typically constitute a significant operational cost for industrial processes, and in many cases, must be recovered and reused to ensure economic feasibility of a process. Although some biocatalytic processes operate using organic solvents, the majority of processes occur in aqueous environments, improving the ease of separation. Most biocatalytic processes occur in batch, differentiating them from conventional chemical processes. As a result, typical bioprocesses employ a separation technique after bioconversion. In this case, product accumulation may cause inhibition of enzyme activity. Ongoing research is performed to develop ''in situ'' separation techniques, where product is removed from the batch during the conversion process. Enzyme separation may be accomplished through solid-liquid extraction techniques such as centrifugation or filtration, and the product-containing solution is fed downstream for product separation.Enzymes as a desired product
To industrialize an enzyme, the following upstream and downstream enzyme production processes are considered:Upstream
Upstream processes are those that contribute to the generation of the enzyme.Selection of a suitable enzyme
An enzyme must be selected based upon the desired reaction. The selected enzyme defines the required operational properties, such as pH, temperature, activity, and substrate affinity.Identification and selection of a suitable source for the selected enzyme
The choice of a source of enzymes is an important step in the production of enzymes. It is common to examine the role of enzymes in nature and how they relate to the desired industrial process. Enzymes are most commonly sourced through bacteria, fungi, and yeast. Once the source of the enzyme is selected, genetic modifications may be performed to increase the expression of the gene responsible for producing the enzyme.Process development
Process development is typically performed after genetic modification of the source organism, and involves the modification of the culture medium and growth conditions. In many cases, process development aims to reduce mRNAhydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
and proteolysis.
Large scale production
Scaling up of enzyme production requires optimization of the fermentation process. Most enzymes are produced under aerobic conditions, and as a result, require constant oxygen input, impacting fermenter design. Due to variations in the distribution of dissolved oxygen, as well as temperature, pH, and nutrients, the transport phenomena associated with these parameters must be considered. The highest possible productivity of the fermenter is achieved at maximum transport capacity of the fermenter.Downstream
Downstream processes are those that contribute to separation or purification of enzymes.Removal of insoluble materials and recovery of enzymes from the source
The procedures for enzyme recovery depend on the source organism, and whether enzymes are intracellular or extracellular. Typically, intracellular enzymes require cell lysis and separation of complex biochemical mixtures. Extracellular enzymes are released into the culture medium, and are much simpler to separate. Enzymes must maintain their native conformation to ensure their catalytic capability. Since enzymes are very sensitive to pH, temperature, and ionic strength of the medium, mild isolation conditions must be used.Concentration and primary purification of enzymes
Depending on the intended use of the enzyme, different levels purity are required. For example, enzymes used for diagnostic purposes must be separated to a higher purity than bulk industrial enzymes to prevent catalytic activity that provides erroneous results. Enzymes used for therapeutic purposes typically require the most rigorous separation. Most commonly, a combination ofchromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
steps is employed for separation.
The purified enzymes are either sold in pure form and sold to other industries, or added to consumer goods.
See also
*Industrial ecology
Industrial ecology (IE) is the study of material and energy flows through industrial systems. The global industrial economy can be modelled as a network of industrial processes that extract resources from the Earth and transform those resource ...
*Industrial fermentation
Industrial fermentation is the intentional use of fermentation in manufacturing products useful to humans. In addition to the mass production of fermented foods and drinks, industrial fermentation has widespread applications in chemical industry. ...
*Industrial microbiology Industrial microbiology is a branch of biotechnology that applies microbial sciences to create industrial products in mass quantities, often using microbial cell factories. There are multiple ways to manipulate a microorganism in order to increase ...
References
{{Reflist