IR spectrum on:
[Wikipedia]
[Google]
[Amazon]
 Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of
 Infrared spectroscopy exploits the fact that molecules absorb frequencies that are characteristic of their structure. These absorptions occur at
Infrared spectroscopy exploits the fact that molecules absorb frequencies that are characteristic of their structure. These absorptions occur at  In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the
In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the
 Simple
Simple

 It is typical to record spectrum of both the sample and a "reference". This step controls for a number of variables, e.g.
It is typical to record spectrum of both the sample and a "reference". This step controls for a number of variables, e.g.
 Fourier transform infrared (FTIR) spectroscopy is a measurement technique that allows one to record infrared spectra. Infrared light is guided through an interferometer and then through the sample (or vice versa). A moving mirror inside the apparatus alters the distribution of infrared light that passes through the interferometer. The signal directly recorded, called an "interferogram", represents light output as a function of mirror position. A data-processing technique called Fourier transform turns this raw data into the desired result (the sample's spectrum): Light output as a function of infrared
Fourier transform infrared (FTIR) spectroscopy is a measurement technique that allows one to record infrared spectra. Infrared light is guided through an interferometer and then through the sample (or vice versa). A moving mirror inside the apparatus alters the distribution of infrared light that passes through the interferometer. The signal directly recorded, called an "interferogram", represents light output as a function of mirror position. A data-processing technique called Fourier transform turns this raw data into the desired result (the sample's spectrum): Light output as a function of infrared
/ref> There are other advantages, as well as some disadvantages, but virtually all modern infrared spectrometers are FTIR instruments.

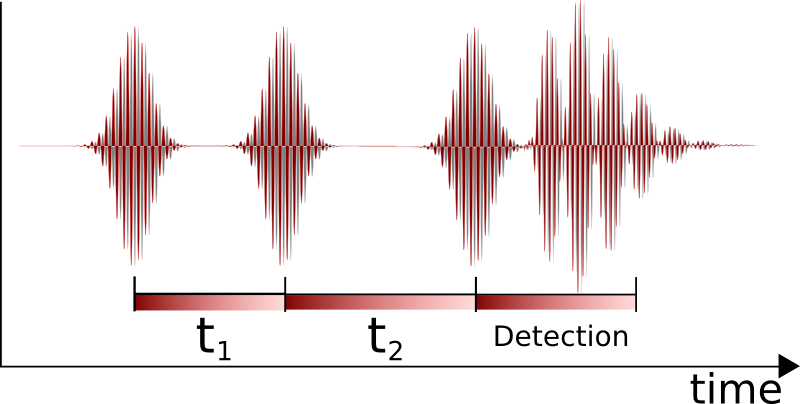 Nonlinear two-dimensional infrared spectroscopy is the infrared version of correlation spectroscopy. Nonlinear two-dimensional infrared spectroscopy is a technique that has become available with the development of femtosecond infrared laser pulses. In this experiment, first a set of pump pulses is applied to the sample. This is followed by a waiting time during which the system is allowed to relax. The typical waiting time lasts from zero to several picoseconds, and the duration can be controlled with a resolution of tens of femtoseconds. A probe pulse is then applied, resulting in the emission of a signal from the sample. The nonlinear two-dimensional infrared spectrum is a two-dimensional correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3 excited by the probe pulse after the waiting time. This allows the observation of coupling between different vibrational modes; because of its extremely fine time resolution, it can be used to monitor molecular dynamics on a picosecond timescale. It is still a largely unexplored technique and is becoming increasingly popular for fundamental research.
As with two-dimensional nuclear magnetic resonance (2DNMR) spectroscopy, this technique spreads the spectrum in two dimensions and allows for the observation of cross peaks that contain information on the coupling between different modes. In contrast to 2DNMR, nonlinear two-dimensional infrared spectroscopy also involves the excitation to overtones. These excitations result in excited state absorption peaks located below the diagonal and cross peaks. In 2DNMR, two distinct techniques, Correlation spectroscopy#COSY, COSY and Correlation spectroscopy#NOESY, NOESY, are frequently used. The cross peaks in the first are related to the scalar coupling, while in the latter they are related to the spin transfer between different nuclei. In nonlinear two-dimensional infrared spectroscopy, analogs have been drawn to these 2DNMR techniques. Nonlinear two-dimensional infrared spectroscopy with zero waiting time corresponds to COSY, and nonlinear two-dimensional infrared spectroscopy with finite waiting time allowing vibrational population transfer corresponds to NOESY. The COSY variant of nonlinear two-dimensional infrared spectroscopy has been used for determination of the secondary structure content of proteins.
Nonlinear two-dimensional infrared spectroscopy is the infrared version of correlation spectroscopy. Nonlinear two-dimensional infrared spectroscopy is a technique that has become available with the development of femtosecond infrared laser pulses. In this experiment, first a set of pump pulses is applied to the sample. This is followed by a waiting time during which the system is allowed to relax. The typical waiting time lasts from zero to several picoseconds, and the duration can be controlled with a resolution of tens of femtoseconds. A probe pulse is then applied, resulting in the emission of a signal from the sample. The nonlinear two-dimensional infrared spectrum is a two-dimensional correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3 excited by the probe pulse after the waiting time. This allows the observation of coupling between different vibrational modes; because of its extremely fine time resolution, it can be used to monitor molecular dynamics on a picosecond timescale. It is still a largely unexplored technique and is becoming increasingly popular for fundamental research.
As with two-dimensional nuclear magnetic resonance (2DNMR) spectroscopy, this technique spreads the spectrum in two dimensions and allows for the observation of cross peaks that contain information on the coupling between different modes. In contrast to 2DNMR, nonlinear two-dimensional infrared spectroscopy also involves the excitation to overtones. These excitations result in excited state absorption peaks located below the diagonal and cross peaks. In 2DNMR, two distinct techniques, Correlation spectroscopy#COSY, COSY and Correlation spectroscopy#NOESY, NOESY, are frequently used. The cross peaks in the first are related to the scalar coupling, while in the latter they are related to the spin transfer between different nuclei. In nonlinear two-dimensional infrared spectroscopy, analogs have been drawn to these 2DNMR techniques. Nonlinear two-dimensional infrared spectroscopy with zero waiting time corresponds to COSY, and nonlinear two-dimensional infrared spectroscopy with finite waiting time allowing vibrational population transfer corresponds to NOESY. The COSY variant of nonlinear two-dimensional infrared spectroscopy has been used for determination of the secondary structure content of proteins.
Infrared spectroscopy for organic chemistsOrganic compounds spectrum database
{{BranchesofSpectroscopy Infrared spectroscopy,
 Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
radiation with matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic part ...
by absorption, emission, or reflection Reflection or reflexion may refer to:
Science and technology
* Reflection (physics), a common wave phenomenon
** Specular reflection, reflection from a smooth surface
*** Mirror image, a reflection in a mirror or in water
** Signal reflection, in ...
. It is used to study and identify chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
s or functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative lo ...
(or transmittance
Transmittance of the surface of a material is its effectiveness in transmitting radiant energy. It is the fraction of incident electromagnetic power that is transmitted through a sample, in contrast to the transmission coefficient, which is t ...
) on the vertical axis vs. frequency
Frequency is the number of occurrences of a repeating event per unit of time. It is also occasionally referred to as ''temporal frequency'' for clarity, and is distinct from ''angular frequency''. Frequency is measured in hertz (Hz) which is eq ...
, wavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the '' spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to te ...
or wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
on the horizontal axis. Typical unit
Unit may refer to:
Arts and entertainment
* UNIT, a fictional military organization in the science fiction television series ''Doctor Who''
* Unit of action, a discrete piece of action (or beat) in a theatrical presentation
Music
* ''Unit'' (a ...
s of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometer Micrometer can mean:
* Micrometer (device), used for accurate measurements by means of a calibrated screw
* American spelling of micrometre
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; ...
s (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal
Reciprocal may refer to:
In mathematics
* Multiplicative inverse, in mathematics, the number 1/''x'', which multiplied by ''x'' gives the product 1, also known as a ''reciprocal''
* Reciprocal polynomial, a polynomial obtained from another pol ...
way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer
A spectrometer () is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the ...
. Two-dimensional IR is also possible as discussed below.
The infrared portion of the electromagnetic spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging fro ...
is usually divided into three regions; the near-, mid- and far- infrared, named for their relation to the visible spectrum. The higher-energy near-IR, approximately 14,000–4,000 cm−1 (0.7–2.5 μm wavelength) can excite overtone
An overtone is any resonant frequency above the fundamental frequency of a sound. (An overtone may or may not be a harmonic) In other words, overtones are all pitches higher than the lowest pitch within an individual sound; the fundamental i ...
or combination modes of molecular vibrations. The mid-infrared, approximately 4,000–400 cm−1 (2.5–25 μm) is generally used to study the fundamental vibrations and associated rotational–vibrational structure. The far-infrared, approximately 400–10 cm−1 (25–1,000 μm) has low energy and may be used for rotational spectroscopy
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave ...
and low frequency vibrations. The region from 2–130 cm−1, bordering the microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ra ...
region, is considered the terahertz region and may probe intermolecular vibrations. The names and classifications of these subregions are conventions, and are only loosely based on the relative molecular or electromagnetic properties.
Theory
resonant frequencies
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillati ...
, i.e. the frequency of the absorbed radiation matches the vibrational frequency. The energies are affected by the shape of the molecular potential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinat ...
s, the masses of the atoms, and the associated vibronic coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" a ...
.
 In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the
In particular, in the Born–Oppenheimer and harmonic approximations, i.e. when the molecular Hamiltonian
In atomic, molecular, and optical physics and quantum chemistry, the molecular Hamiltonian is the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule. This operator and the associated Schrödinger equation p ...
corresponding to the electronic ground state can be approximated by a harmonic oscillator in the neighborhood of the equilibrium molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that dete ...
, the resonant frequencies are associated with the normal modes of vibration corresponding to the molecular electronic ground state potential energy surface.
The resonant frequencies are also related to the strength of the bond and the mass of the atoms at either end of it. Thus, the frequency of the vibrations are associated with a particular normal mode of motion and a particular bond type.
Number of vibrational modes
In order for a vibrational mode in a sample to be "IR active", it must be associated with changes in the dipole moment. A permanent dipole is not necessary, as the rule requires only a change in dipole moment. A molecule can vibrate in many ways, and each way is called a ''vibrational mode.'' For molecules with N number of atoms, geometrically linear molecules have 3''N'' – 5 degrees of vibrational modes, whereas nonlinear molecules have 3''N'' – 6 degrees of vibrational modes (also called vibrational degrees of freedom). As examples linearcarbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
(CO2) has 3 × 3 – 5 = 4, while non-linear water (H2O), has only 3 × 3 – 6 = 3. diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. O ...
s have only one bond and only one vibrational band. If the molecule is symmetrical, e.g. N2, the band is not observed in the IR spectrum, but only in the Raman spectrum
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of Molecule, molecules, although rotational and other low-frequency modes of systems may als ...
. Asymmetrical diatomic molecules, e.g. carbon monoxide ( CO), absorb in the IR spectrum. More complex molecules have many bonds, and their vibrational spectra are correspondingly more complex, i.e. big molecules have many peaks in their IR spectra.
The atoms in a CH2X2 group, commonly found in organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s and where X can represent any other atom, can vibrate in nine different ways. Six of these vibrations involve only the CH2 portion: two stretching modes (ν): symmetric (νs) and antisymmetric (νas); and four bending modes: scissoring (δ), rocking (ρ), wagging (ω) and twisting (τ), as shown below. Structures that do not have the two additional X groups attached have fewer modes because some modes are defined by specific relationships to those other attached groups. For example, in water, the rocking, wagging, and twisting modes do not exist because these types of motions of the H atoms represent simple rotation of the whole molecule rather than vibrations within it. In case of more complex molecules, out-of-plane (γ) vibrational modes can be also present.
These figures do not represent the "recoil
Recoil (often called knockback, kickback or simply kick) is the rearward thrust generated when a gun is being discharged. In technical terms, the recoil is a result of conservation of momentum, as according to Newton's third law the force r ...
" of the C atoms, which, though necessarily present to balance the overall movements of the molecule, are much smaller than the movements of the lighter H atoms.
The simplest and most important or ''fundamental'' IR bands arise from the excitations of normal modes, the simplest distortions of the molecule, from the ground state with vibrational quantum number ''v'' = 0 to the first excited state with vibrational quantum number ''v'' = 1. In some cases, overtone band
In vibrational spectroscopy, an overtone band is the spectral band that occurs in a vibrational spectrum of a molecule when the molecule makes a transition from the ground state (v=0) to the second excited state (v=2), where v is the vibrational q ...
s are observed. An overtone band arises from the absorption of a photon leading to a direct transition from the ground state to the second excited vibrational state (''v'' = 2). Such a band appears at approximately twice the energy of the fundamental band for the same normal mode. Some excitations, so-called ''combination modes'', involve simultaneous excitation of more than one normal mode. The phenomenon of Fermi resonance can arise when two modes are similar in energy; Fermi resonance results in an unexpected shift in energy and intensity of the bands etc.
Practical IR spectroscopy
The infrared spectrum of a sample is recorded by passing a beam of infrared light through the sample. When the frequency of the IR is the same as the vibrational frequency of a bond or collection of bonds, absorption occurs. Examination of the transmitted light reveals how much energy was absorbed at each frequency (or wavelength). This measurement can be achieved by scanning the wavelength range using amonochromator
A monochromator is an optical device that transmits a mechanically selectable narrow band of wavelengths of light or other radiation chosen from a wider range of wavelengths available at the input. The name is from the Greek roots ''mono-'', "s ...
. Alternatively, the entire wavelength range is measured using a Fourier transform instrument and then a transmittance
Transmittance of the surface of a material is its effectiveness in transmitting radiant energy. It is the fraction of incident electromagnetic power that is transmitted through a sample, in contrast to the transmission coefficient, which is t ...
or absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative lo ...
spectrum is generated using a dedicated procedure.
This technique is commonly used for analyzing samples with covalent bonds. Simple spectra are obtained from samples with few IR active bonds and high levels of purity. More complex molecular structures lead to more absorption bands and more complex spectra.

Sample preparation
Gas samples
Gaseous samples require a sample cell with a long pathlength to compensate for the diluteness. The pathlength of the sample cell depends on the concentration of the compound of interest. A simple glass tube with length of 5 to 10 cm equipped with infrared-transparent windows at both ends of the tube can be used for concentrations down to several hundred ppm. Sample gas concentrations well below ppm can be measured with a White's cell in which the infrared light is guided with mirrors to travel through the gas. White's cells are available with optical pathlength starting from 0.5 m up to hundred meters.Liquid samples
Liquid samples can be sandwiched between two plates of a salt (commonly sodium chloride, or common salt, although a number of other salts such as potassium bromide orcalcium fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
...
are also used).
The plates are transparent to the infrared light and do not introduce any lines onto the spectra.
Solid samples
Solid samples can be prepared in a variety of ways. One common method is to crush the sample with an oily mulling agent (usually mineral oil Nujol). A thin film of the mull is applied onto salt plates and measured. The second method is to grind a quantity of the sample with a specially purified salt (usually potassium bromide) finely (to remove scattering effects from large crystals). This powder mixture is then pressed in a mechanicalpress
Press may refer to:
Media
* Print media or news media, commonly called "the press"
* Printing press, commonly called "the press"
* Press (newspaper), a list of newspapers
* Press TV, an Iranian television network
People
* Press (surname), a fam ...
to form a translucent pellet through which the beam of the spectrometer can pass. A third technique is the "cast film" technique, which is used mainly for polymeric materials. The sample is first dissolved in a suitable, non-hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substan ...
solvent. A drop of this solution is deposited on surface of KBr or NaCl
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/ ...
cell. The solution is then evaporated to dryness and the film formed on the cell is analysed directly. Care is important to ensure that the film is not too thick otherwise light cannot pass through. This technique is suitable for qualitative analysis. The final method is to use microtomy to cut a thin (20–100 μm) film from a solid sample. This is one of the most important ways of analysing failed plastic products for example because the integrity of the solid is preserved.
In photoacoustic spectroscopy
Photoacoustic spectroscopy is the measurement of the effect of absorbed electromagnetic energy (particularly of light) on matter by means of acoustic detection. The discovery of the photoacoustic effect dates to 1880 when Alexander Graham Bell sh ...
the need for sample treatment is minimal. The sample, liquid or solid, is placed into the sample cup which is inserted into the photoacoustic cell which is then sealed for the measurement. The sample may be one solid piece, powder or basically in any form for the measurement. For example, a piece of rock can be inserted into the sample cup and the spectrum measured from it.
Comparing to a reference
infrared detector
An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).
The thermal effects of the incident IR radiation can be followed through many temperature depen ...
, which may affect the spectrum. The reference measurement makes it possible to eliminate the instrument influence.
The appropriate "reference" depends on the measurement and its goal. The simplest reference measurement is to simply remove the sample (replacing it by air). However, sometimes a different reference is more useful. For example, if the sample is a dilute solute dissolved in water in a beaker, then a good reference measurement might be to measure pure water in the same beaker. Then the reference measurement would cancel out not only all the instrumental properties (like what light source is used), but also the light-absorbing and light-reflecting properties of the water and beaker, and the final result would just show the properties of the solute (at least approximately).
A common way to compare to a reference is sequentially: first measure the reference, then replace the reference by the sample and measure the sample. This technique is not perfectly reliable; if the infrared lamp is a bit brighter during the reference measurement, then a bit dimmer during the sample measurement, the measurement will be distorted. More elaborate methods, such as a "two-beam" setup (see figure), can correct for these types of effects to give very accurate results. The Standard addition method can be used to statistically cancel these errors.
Nevertheless, among different absorption based techniques which are used for gaseous species detection, Cavity ring-down spectroscopy Cavity ring-down spectroscopy (CRDS) is a highly sensitive optical spectroscopic technique that enables measurement of absolute optical extinction by samples that scatter and absorb light. It has been widely used to study gaseous samples which ab ...
(CRDS) can be used as a calibration free method. The fact that CRDS is based on the measurements of photon life-times (and not the laser intensity) makes it needless for any calibration and comparison with a reference
FTIR
wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
(or equivalently, wavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the '' spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to te ...
). As described above, the sample's spectrum is always compared to a reference.
An alternate method for acquiring spectra is the "dispersive" or "scanning monochromator
A monochromator is an optical device that transmits a mechanically selectable narrow band of wavelengths of light or other radiation chosen from a wider range of wavelengths available at the input. The name is from the Greek roots ''mono-'', "s ...
" method. In this approach, the sample is irradiated sequentially with various single wavelengths. The dispersive method is more common in UV-Vis spectroscopy, but is less practical in the infrared than the FTIR method. One reason that FTIR is favored is called " Fellgett's advantage" or the "multiplex advantage": The information at all frequencies is collected simultaneously, improving both speed and signal-to-noise ratio. Another is called "Jacquinot's Throughput Advantage": A dispersive measurement requires detecting much lower light levels than an FTIR measurement.''Chromatography/Fourier transform infrared spectroscopy and its applications'', by Robert White, p7/ref> There are other advantages, as well as some disadvantages, but virtually all modern infrared spectrometers are FTIR instruments.
Infrared microscopy
Various forms of infrared microscopy exist. These include IR versions of sub-diffraction microscopyH M Pollock and S G Kazarian, Microspectroscopy in the Mid-Infrared, in Encyclopedia of Analytical Chemistry (Robert A. Meyers, Ed, 1-26 (2014), John Wiley & Sons Ltd, such as IR NSOM, photothermal microspectroscopy,Nano-FTIR
Nano-FTIR (nanoscale Fourier transform infrared spectroscopy) is a scanning probe technique that utilizes as a combination of two techniques: Fourier transform infrared spectroscopy (FTIR) and scattering-type scanning near-field optical microsc ...
and AFM-IR, atomic force microscope based infrared spectroscopy (AFM-IR).
Other methods in molecular vibrational spectroscopy
Infrared spectroscopy is not the only method of studying molecular vibrational spectra. Raman spectroscopy involves an inelastic scattering process in which only part of the energy of an incident photon is absorbed by the molecule, and the remaining part is scattered and detected. The energy difference corresponds to absorbed vibrational energy. The selection rules for infrared and for Raman spectroscopy are different at least for some molecular symmetry, molecular symmetries, so that the two methods are complementary in that they observe vibrations of different symmetries. Another method is electron energy loss spectroscopy (EELS), in which the energy absorbed is provided by an inelastically scattered electron rather than a photon. This method is useful for studying vibrations of molecules Adsorption, adsorbed on a solid surface. Recently, High resolution electron energy loss spectroscopy, high-resolution EELS (HREELS) has emerged as a technique for performing vibrational spectroscopy in a Transmission electron microscopy, transmission electron microscope (TEM). In combination with the high spatial resolution of the TEM, unprecedented experiments have been performed, such as nano-scale temperature measurements, mapping of isotopically labeled molecules, mapping of phonon modes in position- and momentum-space, vibrational surface and bulk mode mapping on nanocubes, and investigations of polariton modes in van der Waals crystals. Analysis of vibrational modes that are IR-inactive but appear in inelastic neutron scattering is also possible at high spatial resolution using EELS. Although the spatial resolution of HREELs is very high, the bands are extremely broad compared to other techniques.Computational infrared microscopy
By using computer simulations and normal mode analysis it is possible to calculate theoretical frequencies of molecules.Absorption bands
IR spectroscopy is often used to identify structures becausefunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s give rise to characteristic bands both in terms of intensity and position (frequency). The positions of these bands are summarized in correlation tables as shown below.
Regions
A spectrograph is often interpreted as having two regions. *functional group region In the functional region there are one to a few troughs per functional group. *fingerprint region In the fingerprint region there are many troughs which form an intricate pattern which can be used like a fingerprint to determine the compound.Badger's rule
For many kinds of samples, the assignments are known, i.e. which bond deformation(s) are associated with which frequency. In such cases further information can be gleaned about the strength on a bond, relying on the empirical guideline called Badger's Rule. Originally published by Richard McLean Badger in 1934, this rule states that the strength of a bond (in terms of force constant) correlates with the bond length. That is, increase in bond strength leads to corresponding bond shortening and vice versa.Uses and applications
Infrared spectroscopy is a simple and reliable technique widely used in both organic and inorganic chemistry, in research and industry. In catalysis research it is a very useful tool to characterize the catalyst, as well as to detect intermediates and products during the catalytic reaction. It is used in quality control, dynamic measurement, and monitoring applications such as the long-term unattended measurement of CO2 concentrations in greenhouses and growth chambers by infrared gas analyzers. It is also used in forensic analysis in both criminal and civil cases, for example in identifying polymer degradation. It can be used in determining the blood alcohol content of a suspected drunk driver. IR-spectroscopy has been successfully used in analysis and identification of pigments in paintings and other art objects such as illuminated manuscripts. A useful way of analyzing solid samples without the need for cutting samples uses ATR or attenuated total reflectance spectroscopy. Using this approach, samples are pressed against the face of a single crystal. The infrared radiation passes through the crystal and only interacts with the sample at the interface between the two materials. With increasing technology in computer filtering and manipulation of the results, samples in solution can now be measured accurately (water produces a broad absorbance across the range of interest, and thus renders the spectra unreadable without this computer treatment). Some instruments also automatically identify the substance being measured from a store of thousands of reference spectra held in storage. Infrared spectroscopy is also useful in measuring the degree of polymerization in polymer manufacture. Changes in the character or quantity of a particular bond are assessed by measuring at a specific frequency over time. Modern research instruments can take infrared measurements across the range of interest as frequently as 32 times a second. This can be done whilst simultaneous measurements are made using other techniques. This makes the observations of chemical reactions and processes quicker and more accurate. Infrared spectroscopy has also been successfully utilized in the field of semiconductor microelectronics: for example, infrared spectroscopy can be applied to semiconductors like silicon, gallium arsenide, gallium nitride, zinc selenide, amorphous silicon, silicon nitride, etc. Another important application of Infrared Spectroscopy is in the food industry to measure the concentration of various compounds in different food products. The instruments are now small, and can be transported, even for use in field trials. Infrared Spectroscopy is also used in gas leak detection devices such as the DP-IR and EyeCGAs. These devices detect hydrocarbon gas leaks in the transportation of natural gas and crude oil. In February 2014, NASA announced a greatly upgraded database, based on IR spectroscopy, for tracking polycyclic aromatic hydrocarbons (PAHs) in the universe. According to scientists, more than 20% of the carbon in the universe may be associated with PAHs, possible PAH world hypothesis, starting materials for the Abiogenesis#PAH world hypothesis, formation of Life#Extraterrestrial life, life. PAHs seem to have been formed shortly after the Big Bang, are widespread throughout the universe, and are associated with Star formation, new stars and exoplanets. Infrared spectroscopy is an important analysis method in the recycling process of household waste plastics, and a convenient stand-off method to sort plastic of different polymers (Polyethylene terephthalate, PET, HDPE, ...). Other developments include a miniature IR-spectrometer that's linked to a cloud based database and suitable for personal everyday use, and NIR-spectroscopic chips that can be embedded in smartphones and various gadgets.Isotope effects
The different isotopes in a particular species may exhibit different fine details in infrared spectroscopy. For example, the O–O stretching frequency (in reciprocal centimeters) of oxyhemocyanin is experimentally determined to be 832 and 788 cm−1 for ν(16O–16O) and ν(18O–18O), respectively. By considering the O–O bond as a spring, the frequency of absorbance can be calculated as awavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the '' spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to te ...
[= frequency/(speed of light)]
:
where ''k'' is the spring constant for the bond, ''c'' is the speed of light, and ''μ'' is the reduced mass of the A–B system:
:
( is the mass of atom ).
The reduced masses for 16O–16O and 18O–18O can be approximated as 8 and 9 respectively. Thus
:
The effect of isotopes, both on the vibration and the decay dynamics, has been found to be stronger than previously thought. In some systems, such as silicon and germanium, the decay of the anti-symmetric stretch mode of interstitial oxygen involves the symmetric stretch mode with a strong isotope dependence. For example, it was shown that for a natural silicon sample, the lifetime of the anti-symmetric vibration is 11.4 ps. When the isotope of one of the silicon atoms is increased to 29Si, the lifetime increases to 19 ps. In similar manner, when the silicon atom is changed to 30Si, the lifetime becomes 27 ps.
Two-dimensional IR
Two-dimensional infrared correlation spectroscopy analysis combines multiple samples of infrared spectra to reveal more complex properties. By extending the spectral information of a perturbed sample, spectral analysis is simplified and resolution is enhanced. The 2D synchronous and 2D asynchronous spectra represent a graphical overview of the spectral changes due to a perturbation (such as a changing concentration or changing temperature) as well as the relationship between the spectral changes at two different wavenumbers. Nonlinear two-dimensional infrared spectroscopy is the infrared version of correlation spectroscopy. Nonlinear two-dimensional infrared spectroscopy is a technique that has become available with the development of femtosecond infrared laser pulses. In this experiment, first a set of pump pulses is applied to the sample. This is followed by a waiting time during which the system is allowed to relax. The typical waiting time lasts from zero to several picoseconds, and the duration can be controlled with a resolution of tens of femtoseconds. A probe pulse is then applied, resulting in the emission of a signal from the sample. The nonlinear two-dimensional infrared spectrum is a two-dimensional correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3 excited by the probe pulse after the waiting time. This allows the observation of coupling between different vibrational modes; because of its extremely fine time resolution, it can be used to monitor molecular dynamics on a picosecond timescale. It is still a largely unexplored technique and is becoming increasingly popular for fundamental research.
As with two-dimensional nuclear magnetic resonance (2DNMR) spectroscopy, this technique spreads the spectrum in two dimensions and allows for the observation of cross peaks that contain information on the coupling between different modes. In contrast to 2DNMR, nonlinear two-dimensional infrared spectroscopy also involves the excitation to overtones. These excitations result in excited state absorption peaks located below the diagonal and cross peaks. In 2DNMR, two distinct techniques, Correlation spectroscopy#COSY, COSY and Correlation spectroscopy#NOESY, NOESY, are frequently used. The cross peaks in the first are related to the scalar coupling, while in the latter they are related to the spin transfer between different nuclei. In nonlinear two-dimensional infrared spectroscopy, analogs have been drawn to these 2DNMR techniques. Nonlinear two-dimensional infrared spectroscopy with zero waiting time corresponds to COSY, and nonlinear two-dimensional infrared spectroscopy with finite waiting time allowing vibrational population transfer corresponds to NOESY. The COSY variant of nonlinear two-dimensional infrared spectroscopy has been used for determination of the secondary structure content of proteins.
Nonlinear two-dimensional infrared spectroscopy is the infrared version of correlation spectroscopy. Nonlinear two-dimensional infrared spectroscopy is a technique that has become available with the development of femtosecond infrared laser pulses. In this experiment, first a set of pump pulses is applied to the sample. This is followed by a waiting time during which the system is allowed to relax. The typical waiting time lasts from zero to several picoseconds, and the duration can be controlled with a resolution of tens of femtoseconds. A probe pulse is then applied, resulting in the emission of a signal from the sample. The nonlinear two-dimensional infrared spectrum is a two-dimensional correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3 excited by the probe pulse after the waiting time. This allows the observation of coupling between different vibrational modes; because of its extremely fine time resolution, it can be used to monitor molecular dynamics on a picosecond timescale. It is still a largely unexplored technique and is becoming increasingly popular for fundamental research.
As with two-dimensional nuclear magnetic resonance (2DNMR) spectroscopy, this technique spreads the spectrum in two dimensions and allows for the observation of cross peaks that contain information on the coupling between different modes. In contrast to 2DNMR, nonlinear two-dimensional infrared spectroscopy also involves the excitation to overtones. These excitations result in excited state absorption peaks located below the diagonal and cross peaks. In 2DNMR, two distinct techniques, Correlation spectroscopy#COSY, COSY and Correlation spectroscopy#NOESY, NOESY, are frequently used. The cross peaks in the first are related to the scalar coupling, while in the latter they are related to the spin transfer between different nuclei. In nonlinear two-dimensional infrared spectroscopy, analogs have been drawn to these 2DNMR techniques. Nonlinear two-dimensional infrared spectroscopy with zero waiting time corresponds to COSY, and nonlinear two-dimensional infrared spectroscopy with finite waiting time allowing vibrational population transfer corresponds to NOESY. The COSY variant of nonlinear two-dimensional infrared spectroscopy has been used for determination of the secondary structure content of proteins.
See also
*Applied spectroscopy *Astrochemistry *Atomic and molecular astrophysics *AFM-IR, Atomic force microscopy based infrared spectroscopy (AFM-IR) *Cosmochemistry *Far-infrared astronomy *Forensic chemistry *Forensic engineering *Forensic polymer engineering *Infrared astronomy *Microscopy#Infrared microscopy, Infrared microscopy *Infrared multiphoton dissociation *Infrared photodissociation spectroscopy *Infrared spectroscopy correlation table *Infrared spectroscopy of metal carbonyls *Near-infrared spectroscopy *Nuclear resonance vibrational spectroscopy *Photothermal microspectroscopy *Raman spectroscopy *Rotational-vibrational spectroscopy *Time-resolved spectroscopy *Vibrational spectroscopy of linear moleculesReferences
External links
Infrared spectroscopy for organic chemists
{{BranchesofSpectroscopy Infrared spectroscopy,