Hydrogen spillover on:
[Wikipedia]
[Google]
[Amazon]
 In
In


 In
In heterogeneous catalysis
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. ...
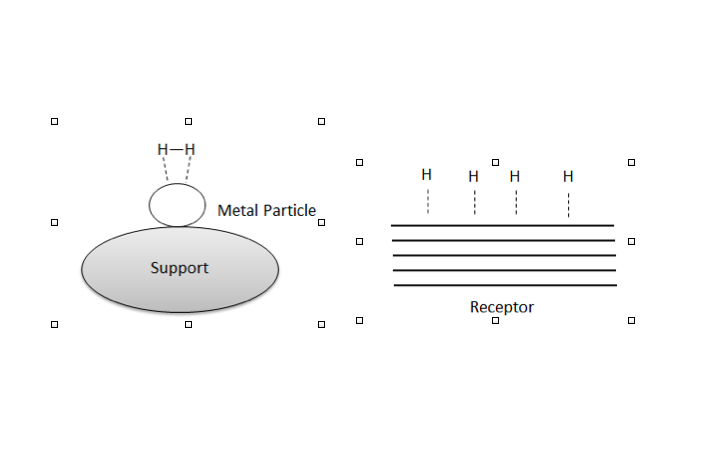
, hydrogen molecules can be adsorbed and dissociated by the metal catalyst. Hydrogen spillover is the migration of hydrogen atoms from the metal catalyst onto the nonmetal support or adsorbate.Gardes, G. E. E., Pajonk, G. M., and S. J. Teichner (1974). “Catalytic Demonstration of Hydrogen Spillover from Nickel-Alumina Catalyst to Alumina.” J. Catal. 33, 145-148. Spillover, generally, is the transport of a species adsorbed or formed on a surface onto another surface.R. Prins: ''Hydrogen Spillover. Facts and Fiction.'' In: ''Chemical Reviews.'' 112, 2012, S. 2714, . Hydrogen spillover can be characterized by three major steps, the first being where molecular hydrogen is split via dissociative chemisorption into its constitutive atoms on a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
catalyst surface, followed by migration from the catalyst to the substrate, culminating in their diffusion throughout the substrate surfaces and/or in the bulk materials.Hansong Cheng, Liang Chen, Alan C. Cooper, Xianwei Sha, Guido P. Pez: ''Hydrogen spillover in the context of hydrogen storage using solid-state materials.'' In: ''Energy & Environmental Science.'' 1, 2008, S. 338, .
Mechanism and trends
Mechanism
Themechanism
Mechanism may refer to:
* Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that ...
behind hydrogen spillover has been long disputed.Sculley, J., Yuan, D., Zhou, H. (2011). “The current status of hydrogen storage in metal–organic frameworks—updated”. Energy Environ. Sci. 4, 2721-2735. Khoobiar’s work in 1964 marks the nascency of the spillover concept. In his findings, yellow WO3 can be reduced by H2 to a blue compound with the use of a platinum catalyst. Since the phenomenon was not found when using Al2O3 as the catalyst, he claimed that the dissociative chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
of H2 molecules on the Pt particles created hydrogen atoms. The hydrogen atoms migrated from the Pt surface to the WO3 particles and reduced them to blue WO3−x particles.
Essentially, hydrogen atoms would migrate from a hydrogen-rich to a hydrogen-poor surface. However, these atoms are usually not generated on the surface of a support metal. Hence, the two conditions for hydrogen spillover include the creation of hydrogen atoms (requires catalysts capable of dissociating and absorbing hydrogen) and the ability of hydrogen atoms to be transported.
Attempts to characterize the mechanism of hydrogen spillover have seen the use of radiation photoelectron spectroscopy to analyze the shift between different oxidation states of the support (commonly metal oxides) via their respective emission spectra.Lykhach, Y., Staudt, T., Vorohkta, M., Skala, T. Johanek, V., Prince, KC., Matolin, V., Libuda, J. (2012). “Hydrogen spillover monitored by resonant photoemission spectroscopy”. J. Catal. 285, 6-9. 12 In general, the mechanism is thought to proceed via the transfer of neutral hydrogen atoms to the support upon overcoming an activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
barrier. This has even been observed at temperatures as low as 180K in metal-organic framework (MOF) catalysts laced with Palladium nanoparticles (PdnP’s). Upon transfer to the support, they assume the role of Lewis bases
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any s ...
where they donate electrons and reversibly reduce
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox react ...
the sorbent. Additionally, the hydrodesulfurization of dibenzothiophene show that hydroxyl groups seem to favor the migration of spillover hydrogen, whereas sodium cations may trap the spillover hydrogen and are detrimental to hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
pathway.Wang, A., Li, X., et al. (2004). “Hydrodesulfurization of Dibenzothiophene Over Proton-Exchanged Siliceous MCM-41 Supported Bimetallic Sulfides”. Dalian University of Technology, China
Recently the mechanism of hydrogen spillover has been described using a precisely nanofabricated model system and single-particle spectromicroscopy. Occurrence of hydrogen spillover on reducible supports such as titanium oxide
Titanium oxide may refer to:
* Titanium dioxide (titanium(IV) oxide), TiO2
* Titanium(II) oxide (titanium monoxide), TiO, a non-stoichiometric oxide
* Titanium(III) oxide (dititanium trioxide), Ti2O3
* Ti3O
* Ti2O
* δ-TiOx (x= 0.68–0.75)
* T ...
is established, yet questions remain about whether hydrogen spillover can take place on nonreducible supports such as aluminium oxide. The study shows a convincing proof of the spillover effect at well-defined distances away from the metal catalyst explaining why hydrogen spillover is slower on an aluminum oxide catalyst support than on a titanium oxide catalyst support. The results reveal that hydrogen spillover is fast and efficient on titanium oxide, and extremely slow and short-ranged on aluminium oxide.

Trends
Hydrogen spillover increases with adsorption temperature and metal dispersion.Andrew, M., and R. Kramer (1979). “Adsorption of Atomic Hydrogen on Alumina by Hydrogen Spillover.” J. Catal. 58, 287-295. A correlation has been reported between available surface area and the capacity forhydrogen storage
Hydrogen storage can be accomplished by several existing methods of holding hydrogen for later use. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand ...
. For PdnP-containing MOFs, in the presence of saturated metal particles, the capacity for hydrogen spillover only relied on the sorbent’s surface area and pore size. On catalysts such as platinum or nickel, atomic hydrogen can be generated at a high frequency. Through surface diffusion, multi-functional transport of hydrogen atoms can enhance a reaction and even regenerate a catalyst. However, problems present in the strength of the hydrogen-support bond; too strong of an interaction would hinder its extraction via reverse spillover and nullify its function as a fuel cell. Conversely, too weak a bond and the hydrogens are easily lost to the environment.

Applications
With burgeoning interest in alternative energy sources, the prospect of hydrogen’s role as a fuel has become a major driving force for the optimization of storage methods, particularly at ambient temperatures where their application would be more practical for common use.Pevzner, S., Pri-Bar, I., Lutzky, I., Ben-Yehuda, E., Ruse, E., Regev, O. (2014). “Carbon Allotropes Accelerate Hydrogenation via Spillover Mechanism”. J. Phys. Chem. C. 118, 27164–27169. Hydrogen spillover has emerged as a possible technique for achieving high-density hydrogen storage at near-ambient conditions in lightweight, solid-state materials as adsorbents.Lueking, A. D., & Yang, R. T. (2004). Hydrogen spillover to enhance hydrogen storage: study of the effect of carbon physicochemical properties. Applied Catalysis A: General, 265, 2.) Hydrogen storage in carbon materials can be significantly enhanced by spillover techniques.Wang, L., & Yang, R. T. (2008). New sorbents for hydrogen storage by hydrogen spillover - a review. Energy & Environmental Science, 1, 2, 268-279Lachawiec, A. J. J., Qi, G., & Yang, R. T. (2005). Hydrogen storage in nanostructured carbons by spillover: bridge-building enhancement. Langmuir : the Acs Journal of Surfaces and Colloids, 21, 24, 11418-24. Current trends include the use of metal-organic frameworks (MOFs) and other porous materials with high surface area for such storage, including but not exclusive to nanocarbons (e.g.graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
, carbon nanotubes
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
), zeolites
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These p ...
, and nanostructured materials. Hydrogen atom diffusion on nanostructured graphitic carbon materials is primarily governed by physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption.
Overview
The fundamental interacting force of physisorption is Van der Waals force. Even ...
of hydrogen atoms. Singled-walled nanotubes and multi-walled nanotubes are the best acceptor of spilt over hydrogen atoms.
Another recent study has shown that the synthesis of methanol from both CO and CO2 over Cu/ZrO2 involves the spillover of H atoms formed on Cu to the surface of ZrO2.Jung, K-D. & Bell, A. T. (2000). “Role of hydrogen spillover in methanol synthesis over Cu/ZrO2”. J. Catal. 193, 207–223 The atomic H then participates in the hydrogenation of carbon-containing species to methanol.
References
{{Authority control Catalysis