Hydrogen fluoride on:
[Wikipedia]
[Google]
[Amazon]
Hydrogen fluoride (fluorane) is an inorganic compound with the
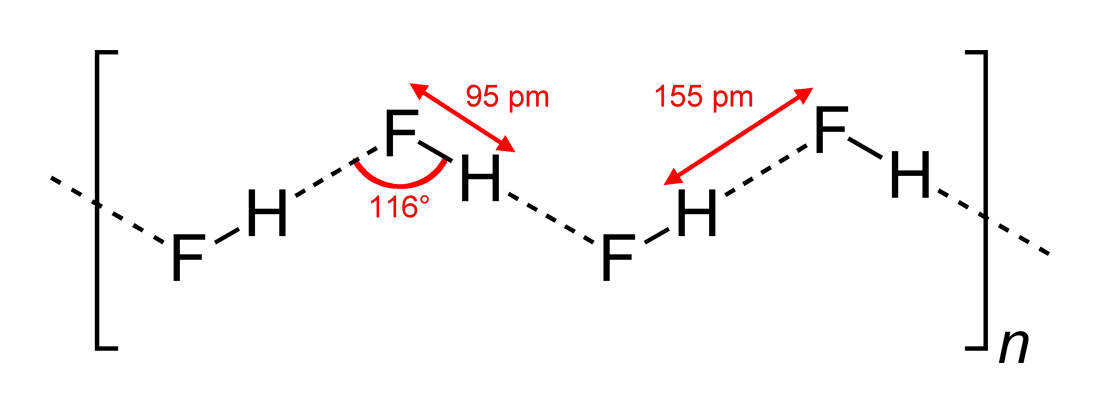 HF is diatomic in the gas-phase. As a liquid, HF forms relatively strong hydrogen bonds, hence its relatively high boiling point. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short covalent H–F bond of 95 pm length, are linked to neighboring molecules by intermolecular H–F distances of 155 pm. Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.
HF is diatomic in the gas-phase. As a liquid, HF forms relatively strong hydrogen bonds, hence its relatively high boiling point. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short covalent H–F bond of 95 pm length, are linked to neighboring molecules by intermolecular H–F distances of 155 pm. Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.
 Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.Facts About Hydrogen Fluoride (Hydrofluoric Acid)
Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.Facts About Hydrogen Fluoride (Hydrofluoric Acid)
/ref> It can cause blindness by rapid destruction of the
Fluorides, Hydrogen Fluoride, and Fluorine
at ATSDR. Retrieved September 30, 2019
CDC - NIOSH Pocket Guide to Chemical HazardsHydrogen Fluoride Fact Sheet
at Toxics Use Reduction Institute {{Authority control Fluorides Hazardous air pollutants Hydrogen compounds Industrial gases Inorganic solvents Nonmetal halides
chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. This colorless gas or liquid is the principal industrial source of fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s, e.g. polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemo ...
(PTFE). HF is widely used in the petrochemical industry
The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics (polymer) industry. It directly interfaces with the petroleum industry, especially the downstream sector.
Comp ...
as a component of superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
s. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides.
Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness
Visual impairment, also known as vision impairment, is a medical definition primarily measured based on an individual's better eye visual acuity; in the absence of treatment such as correctable eyewear, assistive devices, and medical treatment� ...
by rapid destruction of the cornea
The cornea is the transparent front part of the eye that covers the iris, pupil, and anterior chamber. Along with the anterior chamber and lens, the cornea refracts light, accounting for approximately two-thirds of the eye's total optical ...
s.
History
In 1771Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry
Glass production involves two main methods – the float glass process that produces sheet glass, and glassblowing that produces bottles and other containers. It has been done in a variety of ways during the history of glass.
Glass container ...
before then.
French chemist Edmond Frémy (1814–1894) is credited with discovering hydrogen fluoride (HF) while trying to isolate fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
.
Structure and reactions
 HF is diatomic in the gas-phase. As a liquid, HF forms relatively strong hydrogen bonds, hence its relatively high boiling point. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short covalent H–F bond of 95 pm length, are linked to neighboring molecules by intermolecular H–F distances of 155 pm. Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.
HF is diatomic in the gas-phase. As a liquid, HF forms relatively strong hydrogen bonds, hence its relatively high boiling point. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short covalent H–F bond of 95 pm length, are linked to neighboring molecules by intermolecular H–F distances of 155 pm. Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.
Comparison with other hydrogen halides
Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides, which boil between −85 °C (−120 °F) and −35 °C (−30 °F). This hydrogen bonding between HF molecules gives rise to highviscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the int ...
in the liquid phase and lower than expected pressure in the gas phase.
Aqueous solutions
HF is miscible with water (dissolves in any proportion). In contrast, the other hydrogen halides exhibit limiting solubilities in water. Hydrogen fluoride forms a monohydrate HF.H2O with m.p.−40 °C (−40 °F), which is 44 °C (79 °F) above the melting point of pure HF. Aqueous solutions of HF are called hydrofluoric acid. When dilute, hydrofluoric acid behaves like a weak acid, unlike the other hydrohalic acids, due to the formation of hydrogen-bonded ion pairs �F− However concentrated solutions are strong acids, because bifluoride anions are predominant, instead of ion pairs. In liquid anhydrous HF, self-ionization occurs: : which forms an extremely acidic liquid ().Reactions with Lewis acids
Like water, HF can act as a weak base, reacting with Lewis acids to givesuperacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
s. A Hammett acidity function
The Hammett acidity function (''H''0) is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity fu ...
(''H''0) of −21 is obtained with antimony pentafluoride
Antimony pentafluoride is the inorganic compound with the formula Sb F5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, formed when mixing liquid HF with liquid SbF5 in a 2:1 ratio ...
(SbF5), forming fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric ...
.W. L. Jolly "Modern Inorganic Chemistry" (McGraw-Hill 1984), p. 203. . F. A. Cotton and G. Wilkinson, ''Advanced Inorganic Chemistry'' (5th ed.) John Wiley and Sons: New York, 1988. . p. 109.
Production
Hydrogen fluoride is typically produced by the reaction betweensulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
and pure grades of the mineral fluorite:
:
About 20% of manufactured HF is a byproduct of fertilizer production, which generates hexafluorosilicic acid. This acid can be degraded to release HF thermally and by hydrolysis:
:
:
Use
In general, anhydrous hydrogen fluoride is more common industrially than its aqueous solution, hydrofluoric acid. Its main uses, on a tonnage basis, are as a precursor toorganofluorine compound
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refr ...
s and a precursor to cryolite for the electrolysis of aluminium.
Precursor to organofluorine compounds
HF reacts with chlorocarbons to give fluorocarbons. An important application of this reaction is the production oftetrafluoroethylene
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2 F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers.
Properties
Tetrafluoroethylene is a ...
(TFE), precursor to Teflon. Chloroform is fluorinated by HF to produce chlorodifluoromethane (R-22):
:
Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE.
HF is a reactive solvent in the electrochemical fluorination Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds.G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, ...
of organic compounds. In this approach, HF is oxidized in the presence of a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
and the fluorine replaces C–H bonds with C–F bonds. Perfluorinated carboxylic acids and sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is k ...
s are produced in this way.
1,1-Difluoroethane is produced by adding HF to acetylene using mercury as a catalyst.
:
The intermediate in this process is vinyl fluoride
Vinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint etherlike odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride.
Production
It was first prepared in 1901 by F ...
or fluoroethylene, the monomer
In chemistry, a monomer ( ; '' mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
ic precursor to polyvinyl fluoride.
Precursor to metal fluorides and fluorine
The electrowinning ofaluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
relies on the electrolysis of aluminium fluoride in molten cryolite. Several kilograms of HF are consumed per ton of Al produced. Other metal fluorides are produced using HF, including uranium hexafluoride.
HF is the precursor to elemental fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
, F2, by electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of a solution of HF and potassium bifluoride. The potassium bifluoride is needed because anhydrous HF does not conduct electricity. Several thousand tons of F2 are produced annually.
Catalyst
HF serves as acatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
in alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
processes in refineries. It is used in the majority of the installed linear alkyl benzene production facilities in the world. The process involves dehydrogenation of ''n''-paraffins to olefins, and subsequent reaction with benzene using HF as catalyst. For example, in oil refineries
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, li ...
"alkylate", a component of high- octane petrol (gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic c ...
), is generated in alkylation units, which combine C3 and C4 olefins and ''iso''-butane.
Solvent
Hydrogen fluoride is an excellent solvent. Reflecting the ability of HF to participate in hydrogen bonding, even proteins and carbohydrates dissolve in HF and can be recovered from it. In contrast, most non-fluoride inorganic chemicals react with HF rather than dissolving.Greenwood and Earnshaw, "Chemistry of the Elements", pp. 816–819.Health effects
 Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.Facts About Hydrogen Fluoride (Hydrofluoric Acid)
Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.Facts About Hydrogen Fluoride (Hydrofluoric Acid)/ref> It can cause blindness by rapid destruction of the
cornea
The cornea is the transparent front part of the eye that covers the iris, pupil, and anterior chamber. Along with the anterior chamber and lens, the cornea refracts light, accounting for approximately two-thirds of the eye's total optical ...
s. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an irregular heartbeat
Arrhythmias, also known as cardiac arrhythmias, heart arrhythmias, or dysrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults ...
or from pulmonary edema
Pulmonary edema, also known as pulmonary congestion, is excessive liquid accumulation in the tissue and air spaces (usually alveoli) of the lungs. It leads to impaired gas exchange and may cause hypoxemia and respiratory failure. It is due ...
(fluid buildup in the lungs).
References
External links
Fluorides, Hydrogen Fluoride, and Fluorine
at ATSDR. Retrieved September 30, 2019
CDC - NIOSH Pocket Guide to Chemical Hazards
at Toxics Use Reduction Institute {{Authority control Fluorides Hazardous air pollutants Hydrogen compounds Industrial gases Inorganic solvents Nonmetal halides