Glycolaldehyde on:
[Wikipedia]
[Google]
[Amazon]
Glycolaldehyde is the
 In acidic or basic solution, the compound undergoes reversible
In acidic or basic solution, the compound undergoes reversible
 It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and
It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and
 The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in
The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the formula . It is the smallest possible molecule that contains both an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl gro ...
group () and a hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
(). It is a highly reactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
molecule that occurs both in the biosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
and in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
. It is normally supplied as a white solid. Although it conforms to the general formula for carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may o ...
s, , it is not generally considered to be a saccharide.
Structure
Glycolaldehyde as a gas is a simple monomeric structure. As a solid and molten liquid, it exists as adimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
. Collins and George reported the equilibrium of glycolaldehyde in water by using NMR. In aqueous solution, it exists as a mixture of at least four species, which rapidly interconvert.
 In acidic or basic solution, the compound undergoes reversible
In acidic or basic solution, the compound undergoes reversible tautomerization
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
to form 1,2-dihydroxyethene.
It is the only possible diose
A diose is a monosaccharide containing two carbon atoms. Because the general chemical formula of an unmodified monosaccharide is (C·H2O)''n'', where ''n'' is three or greater, it does not meet the formal definition of a monosaccharide. However ...
, a 2-carbon monosaccharide
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water- so ...
, although a diose is not strictly a saccharide. While not a true sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or do ...
, it is the simplest sugar-related molecule. It is reported to taste sweet
Sweetness is a basic taste most commonly perceived when eating foods rich in sugars. Sweet tastes are generally regarded as pleasurable. In addition to sugars like sucrose, many other chemical compounds are sweet, including aldehydes, ketone ...
.
Synthesis
Glycolaldehyde is the second most abundant compound formed when preparingpyrolysis oil
Pyrolysis oil, sometimes also known as bio-crude or bio-oil, is a synthetic fuel under investigation as substitute for petroleum. It is obtained by heating dried biomass without oxygen in a reactor at a temperature of about with subsequent coo ...
(up to 10% by weight).
Glycolaldehyde can be synthesized by the oxidation of ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an o ...
using hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
in the presence of iron(II) sulfate
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the heptahydrate (''x'' = 7) but several values for x are know ...
.
Biosynthesis
It can form by action of ketolase on fructose 1,6-bisphosphate in an alternate glycolysis pathway. This compound is transferred bythiamine pyrophosphate
Thiamine pyrophosphate (TPP or ThPP), or thiamine diphosphate (ThDP), or cocarboxylase is a thiamine (vitamin B1) derivative which is produced by the enzyme thiamine diphosphokinase. Thiamine pyrophosphate is a cofactor that is present in all liv ...
during the pentose phosphate shunt.
In purine catabolism, xanthine
Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffein ...
is first converted to urate. This is converted to 5-hydroxyisourate
5-Hydroxyisourate is a molecule with a formula of C5H4N4O4 and molecular weight of 184.110 g/mol. It is the product of the oxidation of uric acid by urate oxidase.
References
See also
* Urate oxidase
* Glycolaldehyde
Glycolaldehyde is the ...
, which decarboxylates to allantoin
Allantoin is a chemical compound with formula C4H6N4O3. It is also called 5-ureidohydantoin or glyoxyldiureide. It is a diureide of glyoxylic acid. Allantoin is a major metabolic intermediate in most organisms including animals, plants and bacter ...
and allantoic acid
Allantoic acid is an organic compound with the chemical formula C4H8N4O4. It is a crystalline acid obtained by hydrolysis of allantoin.
In nature, allantoic acid is produced from allantoin by the enzyme allantoinase (encoded by the gene AllB (Uni ...
. After hydrolyzing one urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
, this leaves glycolureate. After hydrolyzing the second urea, glycolaldehyde is left. Two glycolaldehydes condense to form erythrose 4-phosphate
Erythrose 4-phosphate is a phosphate of the simple sugar erythrose. It is an intermediate in the pentose phosphate pathway and the Calvin cycle.
In addition, it serves as a precursor in the biosynthesis of the aromatic amino acids tyrosine, pheny ...
, which goes to the pentose phosphate shunt again.
Role in formose reaction
Glycolaldehyde is an intermediate in theformose reaction
The formose reaction, discovered by Aleksandr Butlerov in 1861, and hence also known as the Butlerov reaction, involves the formation of sugars from formaldehyde. The term formose is a portmanteau of formaldehyde and aldose.
Reaction and mechanism ...
. In the formose reaction, two formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
molecules condense to make glycolaldehyde. Glycolaldehyde then is converted to glyceraldehyde
Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3 H6 O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes ...
, presumably via initial tautomerization. The presence of this glycolaldehyde in this reaction demonstrates how it might play an important role in the formation of the chemical building blocks of life. Nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules with ...
, for example, rely on the formose reaction to attain its sugar unit. Nucleotides are essential for life, because they compose the genetic information and coding for life.
Theorized role in abiogenesis
It is often invoked in theories ofabiogenesis
In biology, abiogenesis (from a- 'not' + Greek bios 'life' + genesis 'origin') or the origin of life is the natural process by which life has arisen from non-living matter, such as simple organic compounds. The prevailing scientific hypothes ...
. In the laboratory, it can be converted to amino acids and short dipeptides may have facilitated the formation of complex sugars. For example, L-valyl-L-valine was used as a catalyst to form tetroses from glycolaldehyde. Theoretical calculations have additionally shown the feasibility of dipeptide-catalyzed synthesis of pentoses. This formation showed stereospecific, catalytic synthesis of D-ribose, the only naturally occurring enantiomer of ribose. Since the detection of this organic compound, many theories have been developed related various chemical routes to explain its formation in stellar systems.
 It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and
It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and methyl formate
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other co ...
, the more abundant isomer of glycolaldehyde. The abundances of the products slightly disagree with the observed values found in IRAS 16293-2422, but this can be accounted for by temperature changes. Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an o ...
and glycolaldehyde require temperatures above 30 K. The general consensus among the astrochemistry research community is in favor of the grain surface reaction hypothesis. However, some scientists believe the reaction occurs within denser and colder parts of the core. The dense core will not allow for irradiation as stated before. This change will completely alter the reaction forming glycolaldehyde.
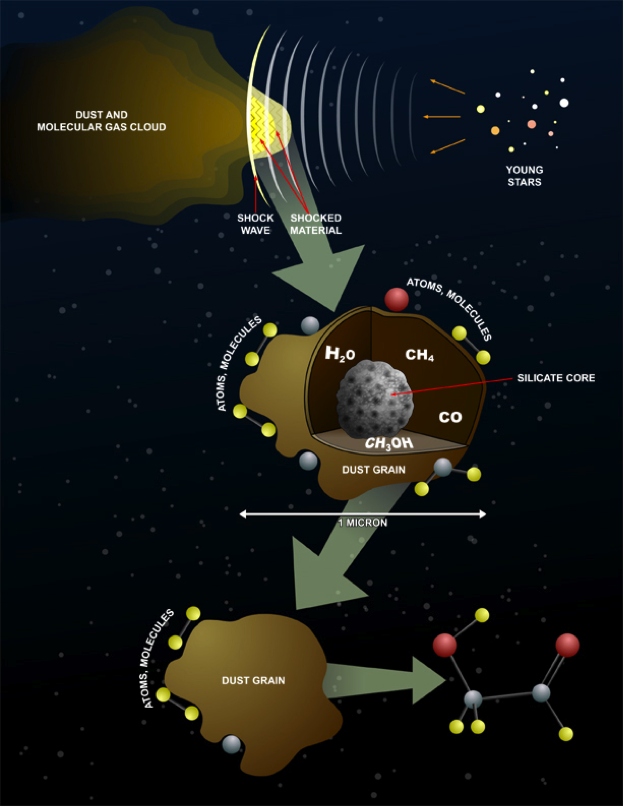
Formation in space
 The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in
The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in cosmic dust
Cosmic dust, also called extraterrestrial dust, star dust or space dust, is dust which exists in outer space, or has fallen on Earth. Most cosmic dust particles measure between a few molecules and 0.1 mm (100 micrometers). Larger particles are c ...
.
Glycolaldehyde has been identified in gas and dust near the center of the Milky Way
The Milky Way is the galaxy that includes our Solar System, with the name describing the galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars that cannot be individually distinguished by the naked eye. ...
galaxy, in a star-forming region 26000 light-years from Earth, and around a protostellar binary star, ''IRAS 16293-2422
The Infrared Astronomical Satellite ( Dutch: ''Infrarood Astronomische Satelliet'') (IRAS) was the first space telescope to perform a survey of the entire night sky at infrared wavelengths. Launched on 25 January 1983, its mission lasted ten mo ...
'', 400 light years from Earth. Observation of in-falling glycolaldehyde spectra 60 AU from ''IRAS 16293-2422'' suggests that complex organic molecules may form in stellar systems prior to the formation of planets, eventually arriving on young planets early in their formation.
Detection in space
The interior region of adust cloud
Fugitive dust is an environmental air quality term for very small particles suspended in the air, primarily mineral dust that is sourced from the soil of Earth's pedosphere. A significant volume of fugitive dust that is visible from a distance i ...
is known to be relatively cold. With temperatures as cold as 4 Kelvin the gases within the cloud will freeze and fasten themselves to the dust, which provides the reaction conditions conducive for the formation of complex molecules such as glycolaldehyde. When a star has formed from the dust cloud, the temperature within the core will increase. This will cause the molecules on the dust to evaporate and be released. The molecule will emit radio waves that can be detected and analyzed. The Atacama Large Millimeter/submillimeter Array
The Atacama Large Millimeter/submillimeter Array (ALMA) is an astronomical interferometer of 66 radio telescopes in the Atacama Desert of northern Chile, which observe electromagnetic radiation at millimeter and submillimeter wavelengths. The ar ...
(ALMA) first detected glycolaldehyde. ALMA consists of 66 antennas that can detect the radio waves emitted from cosmic dust
Cosmic dust, also called extraterrestrial dust, star dust or space dust, is dust which exists in outer space, or has fallen on Earth. Most cosmic dust particles measure between a few molecules and 0.1 mm (100 micrometers). Larger particles are c ...
.
On October 23, 2015, researchers at the Paris Observatory
The Paris Observatory (french: Observatoire de Paris ), a research institution of the Paris Sciences et Lettres University, is the foremost astronomical observatory of France, and one of the largest astronomical centers in the world. Its histo ...
announced the discovery of glycolaldehyde and ethyl alcohol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hyd ...
on Comet Lovejoy, the first such identification of these substances in a comet.
References
External links
* {{Portal bar, Astronomy, Biology Primary alcohols Hydroxy aldehydes