Glutamine synthetase on:
[Wikipedia]
[Google]
[Amazon]
Glutamine synthetase (GS) () is an  Glutamine synthetase uses ammonia produced by nitrate reduction,
Glutamine synthetase uses ammonia produced by nitrate reduction,
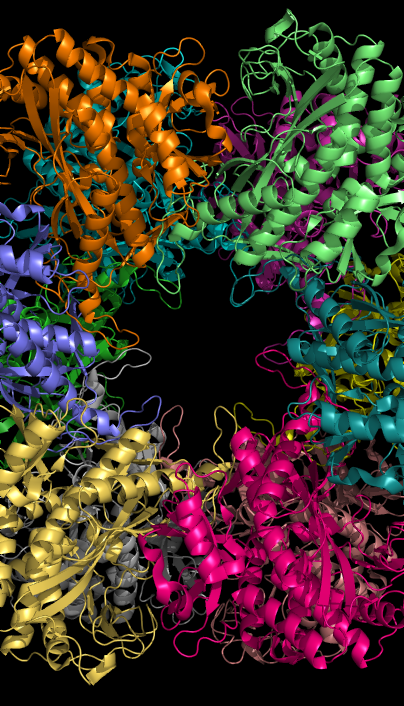 Glutamine synthetase can be composed of 8, 10, or 12 identical subunits separated into two face-to-face rings. Bacterial GS are dodecamers with 12 active sites between each
Glutamine synthetase can be composed of 8, 10, or 12 identical subunits separated into two face-to-face rings. Bacterial GS are dodecamers with 12 active sites between each
 Inhibition of GS has largely focused on amino site ligands. Other inhibitors are the result of glutamine metabolism: tryptophan, histidine, carbamoyl phosphate, glucosamine-6-phosphate, cytidine triphosphate (CTP), and adenosine monophosphate (AMP). Other inhibitors/regulators are glycine and alanine. Alanine, glycine, and serine bind to the glutamate substrate site. GDP, AMP, ADP bind to the ATP site. L-serine, L-alanine, and glycine bind to the site for L-glutamate in unadenylated GS. The four amino acids bind to the site by their common atoms, “the main chain” of amino acids. Glutamate is another product of glutamine metabolism; however, glutamate is a substrate for GS inhibiting it to act as a regulator to GS.2 Each inhibitor can reduce the activity of the enzyme; once all final glutamine metabolites are bound to GS, the activity of GS is almost completely inhibited. Many inhibitory input signals allows for fine tuning of GS by reflecting nitrogen levels in the organism.
Feedback regulation distinguishes the difference between two eukaryotic types of GS: brain and non-brain tissues. Non-brain GS responds to end-product feedback inhibition, while brain GS does not. High concentrations of glutamine-dependent metabolites should inhibit GS activity, while low concentrations should activate GS activity.
Inhibition of GS has largely focused on amino site ligands. Other inhibitors are the result of glutamine metabolism: tryptophan, histidine, carbamoyl phosphate, glucosamine-6-phosphate, cytidine triphosphate (CTP), and adenosine monophosphate (AMP). Other inhibitors/regulators are glycine and alanine. Alanine, glycine, and serine bind to the glutamate substrate site. GDP, AMP, ADP bind to the ATP site. L-serine, L-alanine, and glycine bind to the site for L-glutamate in unadenylated GS. The four amino acids bind to the site by their common atoms, “the main chain” of amino acids. Glutamate is another product of glutamine metabolism; however, glutamate is a substrate for GS inhibiting it to act as a regulator to GS.2 Each inhibitor can reduce the activity of the enzyme; once all final glutamine metabolites are bound to GS, the activity of GS is almost completely inhibited. Many inhibitory input signals allows for fine tuning of GS by reflecting nitrogen levels in the organism.
Feedback regulation distinguishes the difference between two eukaryotic types of GS: brain and non-brain tissues. Non-brain GS responds to end-product feedback inhibition, while brain GS does not. High concentrations of glutamine-dependent metabolites should inhibit GS activity, while low concentrations should activate GS activity.
 Inhibitors:
*
Inhibitors:
*
InterPro entry
PDBe-KB
provides an overview of all the structure information available in the PDB for human glutamine synthetase {{DEFAULTSORT:Glutamine synthetase 2 Glutamate (neurotransmitter)
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
that plays an essential role in the metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run ...
of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
by catalyzing the condensation of glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synt ...
and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
to form glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
:
Glutamate + ATP + NH3 → Glutamine + ADP
Adp or ADP may refer to:
Aviation
* Aéroports de Paris, airport authority for the Parisian region in France
* Aeropuertos del Perú, airport operator for airports in northern Peru
* SLAF Anuradhapura, an airport in Sri Lanka
* Ampara Airp ...
+ phosphate
amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
degradation, and photorespiration
Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reactio ...
. The amide group of glutamate is a nitrogen source for the synthesis of glutamine pathway metabolites.
Other reactions may take place via GS. Competition between ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaterna ...
ion and water, their binding affinities, and the concentration of ammonium ion, influences glutamine synthesis and glutamine hydrolysis. Glutamine is formed if an ammonium ion attacks the acyl-phosphate intermediate, while glutamate is remade if water attacks the intermediate. Ammonium ion binds more strongly than water to GS due to electrostatic forces between a cation and a negatively charged pocket. Another possible reaction is upon NH2OH binding to GS, rather than NH4+, yields γ-glutamylhydroxamate.
Structure
 Glutamine synthetase can be composed of 8, 10, or 12 identical subunits separated into two face-to-face rings. Bacterial GS are dodecamers with 12 active sites between each
Glutamine synthetase can be composed of 8, 10, or 12 identical subunits separated into two face-to-face rings. Bacterial GS are dodecamers with 12 active sites between each monomer
In chemistry, a monomer ( ; '' mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
. Each active site creates a ‘tunnel’ which is the site of three distinct substrate binding sites: nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecu ...
, ammonium ion, and amino acid. ATP binds to the top of the bifunnel that opens to the external surface of GS. Glutamate binds at the bottom of the active site. The middle of the bifunnel contains two sites in which divalent cations bind (Mn+2 or Mg+2). One cation binding site is involved in phosphoryl transfer of ATP to glutamate, while the second stabilizes active GS and helps with the binding of glutamate.
Hydrogen bonding and hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
interactions hold the two rings of GS together. Each subunit possesses a C-terminus and an N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
in its sequence. The C-terminus (helical thong) stabilizes the GS structure by inserting into the hydrophobic region of the subunit across in the other ring. The N-terminus is exposed to the solvent. In addition, the central channel is formed via six four-stranded β-sheets composed of anti-parallel loops from the twelve subunits.
Mechanism
GS catalyzes the ATP-dependent condensation of glutamate with ammonia to yield glutamine. The hydrolysis of ATP drives the first step of a two-part, concerted mechanism. ATP phosphorylates glutamate to form ADP and an acyl-phosphate intermediate, γ-glutamyl phosphate, which reacts with ammonia, forming glutamine and inorganic phosphate. ADP and Pi do not dissociate until ammonia binds and glutamine is released. ATP binds first to the top of the active site near a cation binding site, while glutamate binds near the second cation binding site at the bottom of the active site. The presence of ADP causes a conformational shift in GS that stabilizes the γ-glutamyl phosphate moiety. Ammonium binds strongly to GS only if the acyl-phosphate intermediate is present. Ammonium, rather than ammonia, binds to GS because the binding site is polar and exposed to solvent. In the second step, deprotonation of ammonium allows ammonia to attack the intermediate from its nearby site to form glutamine. Phosphate leaves through the top of the active site, while glutamine leaves through the bottom (between two rings).Biological function
GS is present predominantly in the brain, kidneys, and liver. GS in the brain participates in the metabolic regulation of glutamate, the detoxification of brain ammonia, the assimilation of ammonia, recyclization of neurotransmitters, and termination of neurotransmitter signals. GS, in the brain, is found primarily inastrocytes
Astrocytes (from Ancient Greek , , "star" + , , "cavity", "cell"), also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of ...
. Astrocytes protect neurons against excitotoxicity by taking up excess ammonia and glutamate. In hyperammonemic environments (high levels of ammonia), astroglial swelling occurs. Different perspectives have approached the problem of astroglial swelling. One study shows that morphological changes occur that increase GS expression in glutamatergic areas or other adaptations that alleviates high levels of glutamate and ammonia. Another perspective is that astrocyte swelling is due to glutamine accumulation. To prevent increased levels of cortical glutamate and cortical water content, a study has been conducted to prevent GS activity in rats by the use of MSO.
Classes
There seem to be three different classes of GS: * Class I enzymes (GSI) are specific toprokaryote
A prokaryote () is a single-celled organism that lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek πρό (, 'before') and κάρυον (, 'nut' or 'kernel').Campbell, N. "Biology:Concepts & Con ...
s, and are oligomers of 12 identical subunits. The activity of GSI-type enzyme is controlled by the adenylation of a tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the G ...
residue. The adenylated enzyme is inactive.
* Class II enzymes (GSII) are found in eukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacter ...
and in bacteria belonging to the Rhizobiaceae
The Rhizobiaceae is a family of Pseudomonadota comprising multiple subgroups that enhance and hinder plant development. Some bacteria found in the family are used for plant nutrition and collectively make up the rhizobia. Other bacteria such as ' ...
, Frankiaceae
''Frankia'' is a genus of nitrogen-fixing bacteria that live in symbiosis with actinorhizal plants, similar to the ''Rhizobium'' bacteria found in the root nodules of legumes in the family Fabaceae. ''Frankia'' also initiate the forming of root ...
, and Streptomycetaceae
The ''Streptomycetaceae'' are a family of ''Actinomycetota'', making up the monotypic order ''Streptomycetales''. It includes the important genus ''Streptomyces''. This was the original source of many antibiotics, namely streptomycin, the firs ...
families (these bacteria have also a class-I GS). GSII are decamer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
of identical subunits..
Plants have two or more isozymes of GSII, one of the isozymes is translocated into the chloroplast
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it i ...
. Another form is cytosolic. The cytosolic GS gene translation is regulated by its 5' untranslated region
The 5′ untranslated region (also known as 5′ UTR, leader sequence, transcript leader, or leader RNA) is the region of a messenger RNA (mRNA) that is directly upstream from the initiation codon. This region is important for the regulation of ...
(UTR), while its 3' UTR plays role in transcript turnover.
* Class III enzymes (GSIII) have, currently, only been found in '' Bacteroides fragilis'' and in '' Butyrivibrio fibrisolvens''. It is a double-ringed dodecamer of identical chains. It is much larger (about 700 amino acids) than the GSI (450 to 470 amino acids) or GSII (350 to 420 amino acids) enzymes.
While the three classes of GSs are clearly structurally related, the sequence similarities are not so extensive.
Regulation and inhibition
GS is subject to reversible covalent modification. Tyr397 of all 12 subunits can undergo adenylylation or deadenylylation by adenylyl transferase (AT), a bifunctional regulatory enzyme. Adenylylation is apost-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribo ...
ADP
Adp or ADP may refer to:
Aviation
* Aéroports de Paris, airport authority for the Parisian region in France
* Aeropuertos del Perú, airport operator for airports in northern Peru
* SLAF Anuradhapura, an airport in Sri Lanka
* Ampara Airp ...
. AT activity is influenced by the regulatory protein that is associated with it: PII, a 44-kD trimer. PII also undergoes post-translational modification by uridylyl transferase, thus PII has two forms. The state of PII dictates the activity of adenylyl transferase. If PII is not uridylylated, then it will take on the PIIA form. The AT:PIIA complex will deactivate GS by adenylylation. If PII is uridylylated, then it will take on the PIID form. The AT:PIID complex will activate GS by deadenylylation. The AT:PIIA and AT:PIID complexes are allosterically regulated in a reciprocal fashion by α-ketoglutarate (α-KG) and glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
(Gln). Gln will activate AT:PIIA activity and inhibits AT:PIID, leading to adenylylation and subsequent deactivation of GS. Furthermore, Gln favors the conversion of PIID to PIIA. The effects of α-KG on the complexes are opposite. In the majority of gram-negative bacteria, GS can be modified by adenylylation (some cyanobacteria and green algae or exceptions).
 Inhibition of GS has largely focused on amino site ligands. Other inhibitors are the result of glutamine metabolism: tryptophan, histidine, carbamoyl phosphate, glucosamine-6-phosphate, cytidine triphosphate (CTP), and adenosine monophosphate (AMP). Other inhibitors/regulators are glycine and alanine. Alanine, glycine, and serine bind to the glutamate substrate site. GDP, AMP, ADP bind to the ATP site. L-serine, L-alanine, and glycine bind to the site for L-glutamate in unadenylated GS. The four amino acids bind to the site by their common atoms, “the main chain” of amino acids. Glutamate is another product of glutamine metabolism; however, glutamate is a substrate for GS inhibiting it to act as a regulator to GS.2 Each inhibitor can reduce the activity of the enzyme; once all final glutamine metabolites are bound to GS, the activity of GS is almost completely inhibited. Many inhibitory input signals allows for fine tuning of GS by reflecting nitrogen levels in the organism.
Feedback regulation distinguishes the difference between two eukaryotic types of GS: brain and non-brain tissues. Non-brain GS responds to end-product feedback inhibition, while brain GS does not. High concentrations of glutamine-dependent metabolites should inhibit GS activity, while low concentrations should activate GS activity.
Inhibition of GS has largely focused on amino site ligands. Other inhibitors are the result of glutamine metabolism: tryptophan, histidine, carbamoyl phosphate, glucosamine-6-phosphate, cytidine triphosphate (CTP), and adenosine monophosphate (AMP). Other inhibitors/regulators are glycine and alanine. Alanine, glycine, and serine bind to the glutamate substrate site. GDP, AMP, ADP bind to the ATP site. L-serine, L-alanine, and glycine bind to the site for L-glutamate in unadenylated GS. The four amino acids bind to the site by their common atoms, “the main chain” of amino acids. Glutamate is another product of glutamine metabolism; however, glutamate is a substrate for GS inhibiting it to act as a regulator to GS.2 Each inhibitor can reduce the activity of the enzyme; once all final glutamine metabolites are bound to GS, the activity of GS is almost completely inhibited. Many inhibitory input signals allows for fine tuning of GS by reflecting nitrogen levels in the organism.
Feedback regulation distinguishes the difference between two eukaryotic types of GS: brain and non-brain tissues. Non-brain GS responds to end-product feedback inhibition, while brain GS does not. High concentrations of glutamine-dependent metabolites should inhibit GS activity, while low concentrations should activate GS activity.
Methionine sulfoximine
Methionine sulfoximine (MSO, also known as MetSox) is an irreversible glutamine synthetase inhibitor. It is the sulfoximine derivative of methionine with convulsant effects.
Methionine sulfoximine is composed of two different diastereomers, which ...
(MSO): MSO is an inhibitor that binds to the glutamate site. Bound to GS, MSO is phosphorylated by ATP that results in an irreversible, non-covalent inhibition of GS. The S-isomer configuration is more inhibitory. Glutamate entry is blocked into the active site by a stabilization of the flexible loop in the active site by MSO.
* Phosphinothricin
Glufosinate (also known as phosphinothricin and often sold as an ammonium salt) is a naturally occurring broad-spectrum herbicide produced by several species of ''Streptomyces'' soil bacteria. Glufosinate is a non-selective, contact herbicide, w ...
(PPT, Glufosinate): Phosphinothricin is an inhibitor that binds to the glutamate site. Glufosinate is used as an herbicide. Glufosinate treated plants die due to a buildup of ammonia and a cessation of photosynthesis.
* Many synthetic inhibitors are available today.
Research on '' E. coli'' revealed that GS is regulated through gene expression. The gene that encodes the GS subunit is designated ''glnA''. Transcription of ''glnA'' is dependent on ''NRI'' (a specific transcriptional enhancer). Active transcription occurs if NRI is in its phosphorylated form, designated ''NRI-P''. Phosphorylation of NRI is catalyzed by NRII, a protein kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule don ...
. If NRII is complexed with PIIA then it will function as a phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid monoester into a phosphate ion and an alcohol. Because a phosphatase enzyme catalyzes the hydrolysis of its substrate, it is a subcategory of hydrolase ...
and NRI-P is converted back to NRI. In this case, transcription of ''glnA'' ceases.
GS is subject to completely different regulatory mechanisms in cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, bl ...
. Instead of the common NtrC-NtrB two component system, cyanobacteria harbour the transcriptional regulator NtcA which is restricted to this clade and controls expression of GS and a multitude of genes involved in Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
metabolism. Moreover, GS in Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, bl ...
is not covalently modified to raise sensitivity for feedback inhibition. Instead, GS in Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, bl ...
is inhibited by small proteins, termed GS inactivating factors (IFs) whose transcription is negatively regulated by NtcA. These inactivating factors are furthermore regulated by different Non-coding RNAs: The sRNA NsiR4 interacts with the 5'UTR of the mRNA of the GS inactivating factor IF7 (''gifA'' mRNA) and reduces its expression. NsiR4 expression is under positive control of the nitrogen control transcription factor NtcA. In addition, expression of the GS inactivating factor IF17 is controlled by a glutamine-binding riboswitch.
References
External links
InterPro entry
PDBe-KB
provides an overview of all the structure information available in the PDB for human glutamine synthetase {{DEFAULTSORT:Glutamine synthetase 2 Glutamate (neurotransmitter)