Gilman reagent on:
[Wikipedia]
[Google]
[Amazon]
A Gilman reagent is a

 If the Li+ ions is complexed with the crown ether 12-crown-4, the resulting diorganylcuprate anions adopt a linear coordination geometry at copper.
If the Li+ ions is complexed with the crown ether 12-crown-4, the resulting diorganylcuprate anions adopt a linear coordination geometry at copper.

National Pollutant Inventory - Copper and compounds fact sheet
lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
and copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
( diorganocopper) reagent compound, R2CuLi, where R is an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromaticity, aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar ...
. These reagents are useful because, unlike related Grignard reagents and organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s, they react with Organic halide, organic halides to replace the halides, halide group with an R group (the Corey–House synthesis, Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks.
Reactions
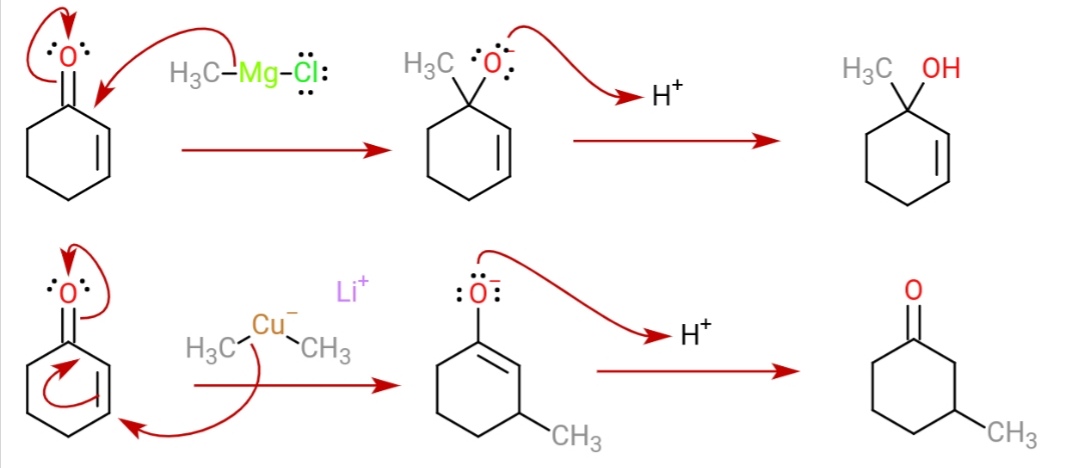
These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic Alpha-beta Unsaturated carbonyl compounds, enone. Due to the softness of the nucleophile, they do 1,4 addition on conjugated enones, rather than 1,2 addition. :
Structure
Lithium dimethylcuprate exists as a Dimer (chemistry), dimer in diethyl ether forming an 8-membered ring. Similarly, lithium diphenylcuprate crystallizes as a dimeric etherate, .



Mixed cuprates
More useful generally than the Gilman reagents are the so-called mixed cuprates with the formula [RCuX]− and [R2CuX]2−. Such compounds are often prepared by the addition of the organolithium reagent to copper(I) halides and cyanide. These mixed cuprates are more stable and more readily purified.Steven H. Bertz, Edward H. Fairchild, Karl Dieter, "Copper(I) Cyanide" in Encyclopedia of Reagents for Organic Synthesis 2005, John Wiley & Sons. One problem addressed by mixed cuprates is the economical use of the alkyl group. Thus, in some applications, the mixed cuprate has the formula is prepared by combining thienyllithium and cuprous cyanide followed by the organic group to be transferred. In this higher order mixed cuprate, both the cyanide and thienyl groups do not transfer, only the R group does.Bruce H. Lipshutz, Robert Moretti, Robert Crow "Mixed Higher-order Cyanocuprate-induced Epoxide Openings: 1-Benzyloxy-4-penten-2-ol" Org. Synth. 1990, volume 69, pp. 80.See also
* Organolithium reagent * Organocopper * Grignard reagent * Cuprate (chemistry)External links
National Pollutant Inventory - Copper and compounds fact sheet
References
{{Lithium compounds Organolithium compounds Organocopper compounds Reagents for organic chemistry