Gas pressure on:
[Wikipedia]
[Google]
[Amazon]
In a mixture of gases, each constituent gas has a partial pressure which is the notional
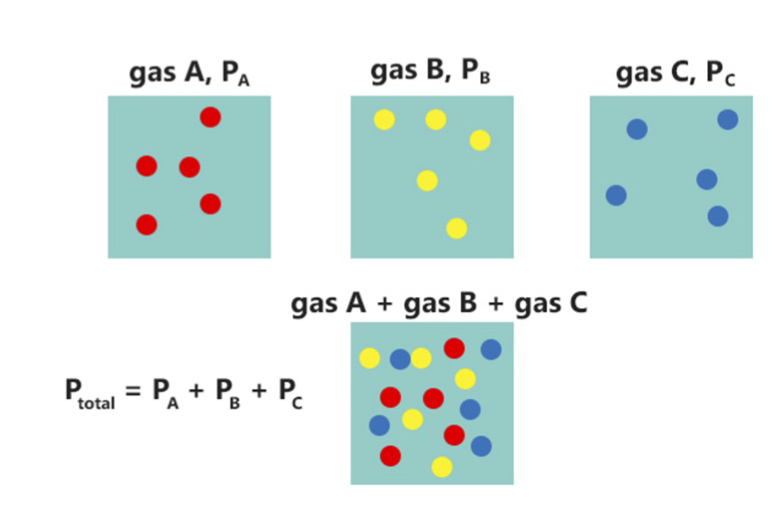 Dalton's law expresses the fact that the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture. This equality arises from the fact that in an ideal gas, the molecules are so far apart that they do not interact with each other. Most actual real-world gases come very close to this ideal. For example, given an ideal gas mixture of
Dalton's law expresses the fact that the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture. This equality arises from the fact that in an ideal gas, the molecules are so far apart that they do not interact with each other. Most actual real-world gases come very close to this ideal. For example, given an ideal gas mixture of
+ <=> +
the equilibrium constant of the reaction would be:
For reversible reactions, changes in the total pressure, temperature or reactant concentrations will shift the equilibrium so as to favor either the right or left side of the reaction in accordance with
/ref> The equilibrium constant for that equilibrium is: where: * = the equilibrium constant for theIntroductory University Chemistry, Henry's Law and the Solubility of Gases
Henry's law is sometimes written as:
where is also referred to as the Henry's law constant. As can be seen by comparing equations () and () above, is the reciprocal of . Since both may be referred to as the Henry's law constant, readers of the technical literature must be quite careful to note which version of the Henry's law equation is being used.
Henry's law is an approximation that only applies for dilute, ideal solutions and for solutions where the liquid solvent does not react chemically with the gas being dissolved.
pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
of that constituent gas as if it alone occupied the entire volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). ...
of the original mixture at the same temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture ( Dalton's Law).
The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', ''number concentration'', ...
s in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathable 20% oxygen and 80% Nitrogen, are determined by volume instead of by weight or mass. Furthermore, the partial pressures of oxygen and carbon dioxide are important parameters in tests of arterial blood gases. That said, these pressures can also be measured in, for example, cerebrospinal fluid
Cerebrospinal fluid (CSF) is a clear, colorless body fluid found within the tissue that surrounds the brain and spinal cord of all vertebrates.
CSF is produced by specialised ependymal cells in the choroid plexus of the ventricles of the ...
.
Symbol
The symbol for pressure is usually or which may use a subscript to identify the pressure, and gas species are also referred to by subscript. When combined, these subscripts are applied recursively. Examples: * or = pressure at time 1 * or = partial pressure of hydrogen * or = venous partial pressure of oxygenDalton's law of partial pressures
 Dalton's law expresses the fact that the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture. This equality arises from the fact that in an ideal gas, the molecules are so far apart that they do not interact with each other. Most actual real-world gases come very close to this ideal. For example, given an ideal gas mixture of
Dalton's law expresses the fact that the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture. This equality arises from the fact that in an ideal gas, the molecules are so far apart that they do not interact with each other. Most actual real-world gases come very close to this ideal. For example, given an ideal gas mixture of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
(N2), hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
(H2) and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
(NH3):
where:
* = total pressure of the gas mixture
* = partial pressure of nitrogen (N2)
* = partial pressure of hydrogen (H2)
* = partial pressure of ammonia (NH3)
Ideal gas mixtures
Ideally the ratio of partial pressures equals the ratio of the number of molecules. That is, the mole fraction of an individual gas component in an ideal gasmixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
can be expressed in terms of the component's partial pressure or the moles Moles can refer to:
* Moles de Xert, a mountain range in the Baix Maestrat comarca, Valencian Community, Spain
*The Moles (Australian band)
*The Moles, alter ego of Scottish band Simon Dupree and the Big Sound
People
* Abraham Moles, French engin ...
of the component:
and the partial pressure of an individual gas component in an ideal gas can be obtained using this expression:
The mole fraction of a gas component in a gas mixture is equal to the volumetric fraction of that component in a gas mixture.
The ratio of partial pressures relies on the following isotherm relation:
* ''V''X is the partial volume of any individual gas component (X)
* ''V''tot is the total volume of the gas mixture
* ''p''X is the partial pressure of gas X
* ''p''tot is the total pressure of the gas mixture
* ''n''X is the amount of substance
In chemistry, the amount of substance ''n'' in a given sample of matter is defined as the quantity or number of discrete atomic-scale particles in it divided by the Avogadro constant ''N''A. The particles or entities may be molecules, atoms, io ...
of gas (X)
* ''n''tot is the total amount of substance in gas mixture
Partial volume (Amagat's law of additive volume)
The partial volume of a particular gas in a mixture is the volume of one component of the gas mixture. It is useful in gas mixtures, e.g. air, to focus on one particular gas component, e.g. oxygen. It can be approximated both from partial pressure and molar fraction:Page 200 in: Medical biophysics. Flemming Cornelius. 6th Edition, 2008. * ''V''X is the partial volume of an individual gas component X in the mixture * ''V''tot is the total volume of the gas mixture * ''p''X is the partial pressure of gas X * ''p''tot is the total pressure of the gas mixture * ''n''X is theamount of substance
In chemistry, the amount of substance ''n'' in a given sample of matter is defined as the quantity or number of discrete atomic-scale particles in it divided by the Avogadro constant ''N''A. The particles or entities may be molecules, atoms, io ...
of gas X
* ''n''tot is the total amount of substance in the gas mixture
Vapor pressure
Vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed pha ...
is the pressure of a vapor in equilibrium with its non-vapor phases (i.e., liquid or solid). Most often the term is used to describe a liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, ...
's tendency to evaporate
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humi ...
. It is a measure of the tendency of molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s and atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
s to escape from a liquid or a solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structur ...
. A liquid's atmospheric pressure boiling point corresponds to the temperature at which its vapor pressure is equal to the surrounding atmospheric pressure and it is often called the normal boiling point.
The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point of the liquid.
The vapor pressure chart displayed has graphs of the vapor pressures versus temperatures for a variety of liquids. As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points.
For example, at any given temperature, methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industria ...
has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point (−24.2 °C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere ( atm) of absolute vapor pressure. Note that at higher altitudes, the atmospheric pressure is less than that at sea level, so boiling points of liquids are reduced. At the top of Mount Everest
Mount Everest (; Tibetan: ''Chomolungma'' ; ) is Earth's highest mountain above sea level, located in the Mahalangur Himal sub-range of the Himalayas. The China–Nepal border runs across its summit point. Its elevation (snow hei ...
, the atmospheric pressure is approximately 0.333 atm, so by using the graph, the boiling point of diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
would be approximately 7.5 °C versus 34.6 °C at sea level (1 atm).
Equilibrium constants of reactions involving gas mixtures
It is possible to work out theequilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for a chemical reaction involving a mixture of gases given the partial pressure of each gas and the overall reaction formula. For a reversible reaction involving gas reactants and gas products, such as:
Le Chatelier's Principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French ...
. However, the reaction kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in w ...
may either oppose or enhance the equilibrium shift. In some cases, the reaction kinetics may be the overriding factor to consider.
Henry's law and the solubility of gases
Gases will dissolve inliquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, ...
s to an extent that is determined by the equilibrium between the undissolved gas and the gas that has dissolved in the liquid (called the ''solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
'').An extensive list of Henry's law constants, and a conversion tool/ref> The equilibrium constant for that equilibrium is: where: * = the equilibrium constant for the
solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of t ...
process
* = partial pressure of gas in equilibrium with a solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Solutio ...
containing some of the gas
* = the concentration of gas in the liquid solution
The form of the equilibrium constant shows that the concentration of a solute gas in a solution is directly proportional to the partial pressure of that gas above the solution. This statement is known as Henry's law and the equilibrium constant is quite often referred to as the Henry's law constant.In diving breathing gases
In underwater diving the physiological effects of individual component gases of breathing gases are a function of partial pressure. Using diving terms, partial pressure is calculated as: :partial pressure = (total absolute pressure) × (volume fraction of gas component) For the component gas "i": :pi = P × Fi For example, at underwater, the total absolute pressure is (i.e., 1 bar of atmospheric pressure + 5 bar of water pressure) and the partial pressures of the main components of air,oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
21% by volume and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
approximately 79% by volume are:
:pN2 = 6 bar × 0.79 = 4.7 bar absolute
:pO2 = 6 bar × 0.21 = 1.3 bar absolute
The minimum safe lower limit for the partial pressures of oxygen in a breathing gas mixture for diving is absolute. Hypoxia and sudden unconsciousness can become a problem with an oxygen partial pressure of less than 0.16 bar absolute. Oxygen toxicity
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen () at increased partial pressures. Severe cases can result in cell damage and death, with effects most often seen in the central nervous system, l ...
, involving convulsions, becomes a problem when oxygen partial pressure is too high. The NOAA
The National Oceanic and Atmospheric Administration (abbreviated as NOAA ) is an United States scientific and regulatory agency within the United States Department of Commerce that forecasts weather, monitors oceanic and atmospheric conditio ...
Diving Manual recommends a maximum single exposure of 45 minutes at 1.6 bar absolute, of 120 minutes at 1.5 bar absolute, of 150 minutes at 1.4 bar absolute, of 180 minutes at 1.3 bar absolute and of 210 minutes at 1.2 bar absolute. Oxygen toxicity becomes a risk when these oxygen partial pressures and exposures are exceeded. The partial pressure of oxygen also determines the maximum operating depth of a gas mixture.
Narcosis is a problem when breathing gases at high pressure. Typically, the maximum total partial pressure of narcotic gases used when planning for technical diving
Technical diving (also referred to as tec diving or tech diving) is scuba diving that exceeds the agency-specified limits of recreational diving for non- professional purposes. Technical diving may expose the diver to hazards beyond those normal ...
may be around 4.5 bar absolute, based on an equivalent narcotic depth
Equivalent narcotic depth (END) is used in technical diving as a way of estimating the narcotic effect of a breathing gas
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and ...
of .
The effect of a toxic contaminant such as carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
in breathing gas is also related to the partial pressure when breathed. A mixture which may be relatively safe at the surface could be dangerously toxic at the maximum depth of a dive, or a tolerable level of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
in the breathing loop of a diving rebreather
A Diving rebreather is an underwater breathing apparatus that absorbs the carbon dioxide of a diver's exhaled breath to permit the rebreathing (recycling) of the substantially unused oxygen content, and unused inert content when present, of ea ...
may become intolerable within seconds during descent when the partial pressure rapidly increases, and could lead to panic or incapacitation of the diver.
In medicine
The partial pressures of particularly oxygen () and carbon dioxide () are important parameters in tests of arterial blood gases, but can also be measured in, for example,cerebrospinal fluid
Cerebrospinal fluid (CSF) is a clear, colorless body fluid found within the tissue that surrounds the brain and spinal cord of all vertebrates.
CSF is produced by specialised ependymal cells in the choroid plexus of the ventricles of the ...
.
See also
* * * ** * ** *References
{{authority control Engineering thermodynamics Equilibrium chemistry Gas laws Gases Physical chemistry Pressure Underwater diving physics Distillation