SuperCool 2009-01-02.ogv on:
[Wikipedia]
[Google]
[Amazon]

 Supercooling, also known as undercooling, is the process of lowering the temperature of a
Supercooling, also known as undercooling, is the process of lowering the temperature of a
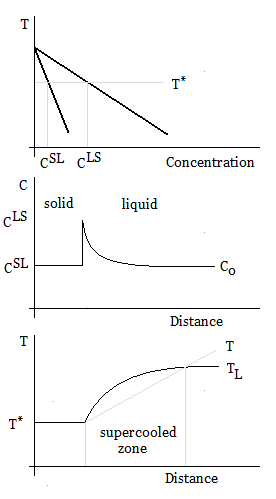 Constitutional supercooling, which occurs during solidification, is due to compositional solid changes, and results in cooling a liquid below the freezing point ahead of the solid–liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid–liquid interface must be small in order to avoid constitutional supercooling.
Constitutional supercooling is observed when the
Constitutional supercooling, which occurs during solidification, is due to compositional solid changes, and results in cooling a liquid below the freezing point ahead of the solid–liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid–liquid interface must be small in order to avoid constitutional supercooling.
Constitutional supercooling is observed when the
Supercooled liquids on arxiv.orgRadiolab podcast on supercooling
{{Authority control Thermodynamic processes Condensed matter physics Concepts in physics Glass physics

 Supercooling, also known as undercooling, is the process of lowering the temperature of a
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−55 °F). Droplets of supercooled water often exist in stratus and cumulus cloud
Cumulus clouds are clouds which have flat bases and are often described as "puffy", "cotton-like" or "fluffy" in appearance. Their name derives from the Latin ''cumulo-'', meaning ''heap'' or ''pile''. Cumulus clouds are low-level clouds, gener ...
s. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes.
Animals utilize supercooling to survive in extreme temperatures. There are many mechanisms that aid in maintaining a liquid state, such as the production of antifreeze proteins, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice. The winter flounder is one such fish that utilizes these proteins to survive in its frigid environment. In plants, cellular barriers such as lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity ...
, suberin and the cuticle inhibit ice nucleators and force water into the supercooled tissue.
One commercial application of supercooling is in refrigeration. Freezers can cool drinks to a supercooled level so that when they are opened, they form a slush
Slush, also called slush ice, is a slurry mixture of small ice crystals (e.g., snow) and liquid water.
In the natural environment, slush forms when ice or snow melts or during mixed precipitation. This often mixes with dirt and other pollutant ...
. Supercooling was also successfully applied to organ preservation at Massachusetts General Hospital
Massachusetts General Hospital (Mass General or MGH) is the original and largest teaching hospital of Harvard Medical School located in the West End neighborhood of Boston, Massachusetts. It is the third oldest general hospital in the United Stat ...
/ Harvard Medical School. Livers that were later transplanted into recipient animals were preserved by supercooling for up to 96 hours (4 days), quadrupling the limits of what could be achieved by conventional liver preservation methods.
Explanation
A liquid crossing its standard freezing point will crystalize in the presence of a seed crystal or nucleus around which a crystal structure can form creating a solid. Lacking any such nuclei, the liquidphase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
* Phase space, a mathematic ...
can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs.
Homogeneous nucleation can occur above the glass transition temperature, but if homogeneous nucleation has not occurred above that temperature, an amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
(non-crystalline) solid will form.
Water normally freezes at 273.15 K (0 °C or 32 °F), but it can be "supercooled" at standard pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
down to its crystal homogeneous nucleation at almost 224.8 K (−48.3 °C/−55 °F). The process of supercooling requires that water be pure and free of nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that deter ...
sites, which can be achieved by processes like reverse osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pre ...
or chemical demineralization, but the cooling itself does not require any specialised technique. If water is cooled at a rate on the order of 106 K/s, the crystal nucleation can be avoided and water becomes a glass—that is, an amorphous (non-crystalline) solid. Its glass transition temperature is much colder and harder to determine, but studies estimate it at about 136 K (−137 °C/−215 °F).
Glassy water
Amorphous ice (non-crystalline or "vitreous" ice) is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice has a lack of long-range order ...
can be heated up to approximately 150 K (−123 °C/−189.4 °F) without nucleation occurring.
In the range of temperatures between 231 K (−42 °C/−43.6 °F) and 150 K (−123 °C/−189.4 °F), experiments find only crystal ice.
Droplets of supercooled water often exist in stratus and cumulus cloud
Cumulus clouds are clouds which have flat bases and are often described as "puffy", "cotton-like" or "fluffy" in appearance. Their name derives from the Latin ''cumulo-'', meaning ''heap'' or ''pile''. Cumulus clouds are low-level clouds, gener ...
s. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes, unless the aircraft is equipped with an appropriate de-icing system. Freezing rain is also caused by supercooled droplets.
The process opposite to supercooling, the melting of a solid above the freezing point, is much more difficult, and a solid will almost always melt at the same temperature for a given pressure. For this reason, it is the melting point which is usually identified, using melting point apparatus
A melting-point apparatus is a scientific instrument used to determine the melting point of a substance. Some types of melting-point apparatuses include the Thiele tube, Fisher-Johns apparatus, Gallenkamp (Electronic) melting-point apparatus and ...
; even when the subject of a paper is "freezing-point determination", the actual methodology is "the principle of observing the disappearance rather than the formation of ice". It is possible, at a given pressure, to superheat a liquid above its boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
without it becoming gaseous.
Supercooling should not be confused with freezing-point depression. Supercooling is the cooling of a liquid below its freezing point without it becoming solid. Freezing point depression is when a solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
can be cooled below the freezing point of the corresponding pure liquid due to the presence of the solute; an example of this is the freezing point depression that occurs when salt is added to pure water.
Constitutional supercooling
 Constitutional supercooling, which occurs during solidification, is due to compositional solid changes, and results in cooling a liquid below the freezing point ahead of the solid–liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid–liquid interface must be small in order to avoid constitutional supercooling.
Constitutional supercooling is observed when the
Constitutional supercooling, which occurs during solidification, is due to compositional solid changes, and results in cooling a liquid below the freezing point ahead of the solid–liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid–liquid interface must be small in order to avoid constitutional supercooling.
Constitutional supercooling is observed when the liquidus
The liquidus temperature, TL or Tliq, specifies the temperature above which a material is completely liquid, and the maximum temperature at which crystals can co-exist with the melt in thermodynamic equilibrium. It is mostly used for impure subst ...
temperature gradient at the interface (the position x=0) is larger than the imposed temperature gradient:
:
The liquidus slope from the binary phase diagram is given by , so the constitutional supercooling criterion for a binary alloy can be written in terms of the concentration gradient at the interface:
:
The concentration gradient ahead of a planar interface is given by
:
where is the interface velocity, the diffusion coefficient
Diffusivity, mass diffusivity or diffusion coefficient is a proportionality constant between the molar flux due to molecular diffusion and the gradient in the concentration of the species (or the driving force for diffusion). Diffusivity is enco ...
, and and are the compositions of the liquid and solid at the interface, respectively (i.e., ).
For the steady-state growth of a planar interface, the composition of the solid is equal to the nominal alloy composition, , and the partition coefficient, , can be assumed constant. Therefore, the minimum thermal gradient necessary to create a stable solid front is given by
:
For more information, see Chapter 3 of
In animals
In order to survive extreme low temperatures in certain environments, some animals use the phenomenon of supercooling that allow them to remain unfrozen and avoid cell damage and death. There are many techniques that aid in maintaining a liquid state, such as the production of antifreeze proteins, or AFPs, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice. The winter flounder is one such fish that utilizes these proteins to survive in its frigid environment. Noncolligative proteins are secreted by the liver into the bloodstream. Other animals use colligative antifreezes, which increases the concentration of solutes in their bodily fluids, thus lowering their freezing point. Fish that rely on supercooling for survival must also live well below the water surface, because if they came into contact with ice nuclei they would freeze immediately. Animals that undergo supercooling to survive must also remove ice-nucleating agents from their bodies because they act as a starting point for freezing. Supercooling is also a common feature in some insect, reptile, and other ectotherm species. The potato cyst nematode larva ('' Globodera rostochiensis'') could survive inside their cysts in a supercooled state to temperatures as low as , even with the cyst encased in ice. As an animal gets farther and farther below its melting point the chance of spontaneous freezing increases dramatically for its internal fluids, as this is a thermodynamically unstable state. The fluids eventually reach the supercooling point, which is the temperature at which the supercooled solution freezes spontaneously due to being so far below its normal freezing point. Animals unintentionally undergo supercooling and are only able to decrease the odds of freezing once supercooled. Even though supercooling is essential for survival, there are many risks associated with it.In plants
Plants can also survive extreme cold conditions brought forth during the winter months. Many plant species located in northern climates can acclimate under these cold conditions by supercooling, thus these plants survive temperatures as low as −40 °C. Although this supercooling phenomenon is poorly understood, it has been recognized through infrared thermography. Ice nucleation occurs in certain plant organs and tissues, debatably beginning in the xylem tissue and spreading throughout the rest of the plant. Infrared thermography allows for droplets of water to be visualized as they crystalize in extracellular spaces. Supercooling inhibits the formation of ice within the tissue by ice nucleation and allows the cells to maintain water in a liquid state and further allows the water within the cell to stay separate from extracellular ice. Cellular barriers such aslignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity ...
, suberin and the cuticle inhibit ice nucleators and force water into the supercooled tissue. The xylem and primary tissue of plants are very susceptible to cold temperatures because of the large proportion of water in the cell. Many boreal hardwood species in northern climates have the ability to prevent ice spreading into the shoots allowing the plant to tolerate the cold. Supercooling has been identified in the evergreen shrubs ''Rhododendron ferrugineum
''Rhododendron ferrugineum'', the alpenrose, snow-rose, or rusty-leaved alpenrose is an evergreen shrub that grows just above the tree line in the Alps, Pyrenees, Jura and northern Apennines, on acid soils. It is the type species for the genus ' ...
'' and '' Vaccinium vitis-idaea'' as well as '' Abies'', ''Picea
A spruce is a tree of the genus ''Picea'' (), a genus of about 35 species of coniferous evergreen trees in the family Pinaceae, found in the northern temperate and boreal (taiga) regions of the Earth. ''Picea'' is the sole genus in the subfami ...
'' and ''Larix
Larches are deciduous conifers in the genus ''Larix'', of the family Pinaceae (subfamily Laricoideae). Growing from tall, they are native to much of the cooler temperate northern hemisphere, on lowlands in the north and high on mountains furth ...
'' species. Freezing outside of the cell and within the cell wall does not affect the survival of the plant. However, the extracellular ice may lead to plant dehydration.
In seawater
The presence of salt in seawater affects the freezing point. For that reason, it is possible for seawater to remain in the liquid state at temperatures below melting point. This is "pseudo-supercooling" because the phenomena is the result of freezing point lowering caused by the presence of salt, not supercooling. This condition is most commonly observed in the oceans around Antarctica where melting of the undersides of ice shelves at high pressure results in liquid melt-water that can be below the freezing temperature. It is supposed that the water does not immediately refreeze due to a lack of nucleation sites. This provides a challenge to oceanographic instrumentation as ice crystals will readily form on the equipment, potentially affecting the data quality. Ultimately the presence of extremely cold seawater will affect the growth ofsea ice
Sea ice arises as seawater freezes. Because ice is less dense than water, it floats on the ocean's surface (as does fresh water ice, which has an even lower density). Sea ice covers about 7% of the Earth's surface and about 12% of the world's oce ...
.
Applications
One commercial application of supercooling is in refrigeration. Freezers can cool drinks to a supercooled level so that when they are opened, they form aslush
Slush, also called slush ice, is a slurry mixture of small ice crystals (e.g., snow) and liquid water.
In the natural environment, slush forms when ice or snow melts or during mixed precipitation. This often mixes with dirt and other pollutant ...
. Another example is a product that can supercool the beverage in a conventional freezer. The Coca-Cola Company briefly marketed special vending machines containing Sprite in the UK, and Coke in Singapore, which stored the bottles in a supercooled state so that their content would turn to slush
Slush, also called slush ice, is a slurry mixture of small ice crystals (e.g., snow) and liquid water.
In the natural environment, slush forms when ice or snow melts or during mixed precipitation. This often mixes with dirt and other pollutant ...
upon opening.
Supercooling was successfully applied to organ preservation at Massachusetts General Hospital/ Harvard Medical School. Livers that were later transplanted into recipient animals were preserved by supercooling for up to 96 hours (4 days), quadrupling the limits of what could be achieved by conventional liver preservation methods. The livers were supercooled to a temperature of –6 °C in a specialized solution that protected against freezing and injury from the cold temperature.
Another potential application is drug delivery. In 2015, researchers crystallized membranes at a specific time. Liquid-encapsulated drugs could be delivered to the site and, with a slight environmental change, the liquid rapidly changes into a crystalline form that releases the drug.
In 2016, a team at Iowa State University proposed a method for "soldering without heat" by using encapsulated droplets of supercooled liquid metal to repair heat sensitive electronic devices. In 2019, the same team demonstrated the use of undercooled metal to print solid metallic interconnects on surfaces ranging from polar (paper and Jello) to superhydrophobic (rose petals), with all the surfaces being lower modulus than the metal.
Eftekhari et al. proposed an empirical theory explaining that supercooling of ionic liquid crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. T ...
s can build ordered channels for diffusion for energy storage applications. In this case, the electrolyte has a rigid structure comparable with that of a solid electrolyte, but the diffusion coefficient can be as large as in liquid electrolytes. Supercooling increases the medium viscosity but keeps the directional channels open for diffusion.
In spaceflight
In spaceflight applications, the term is used somewhat differently. Here it refers tocryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
fuels or oxidizers which are cooled well below their ''boiling'' point (but not below the ''melting'' point). This results in a higher fuel density, and hence a higher capacity of the fuel tanks without increasing their weight. At the same time vaporization losses are reduced.
SpaceX
Space Exploration Technologies Corp. (SpaceX) is an American spacecraft manufacturer, launcher, and a satellite communications corporation headquartered in Hawthorne, California. It was founded in 2002 by Elon Musk with the stated goal of ...
's Falcon 9 rocket uses supercooling for its oxidizer.
The term ''superchilling'' is also used for this technique.
See also
* Amorphous solid * Pumpable ice technology * Subcooling * Ultracold atom *Viscous liquid
In condensed matter physics and physical chemistry, the terms viscous liquid, supercooled liquid, and glassforming liquid are often used interchangeably to designate liquids that are at the same time highly viscous (see Viscosity of amorphous mate ...
* Freezing rain
References
Further reading
* *External links
* * * *Supercooled liquids on arxiv.org
{{Authority control Thermodynamic processes Condensed matter physics Concepts in physics Glass physics