Oxygen molecule orbitals diagram.JPG on:
[Wikipedia]
[Google]
[Amazon]
Oxygen is the
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol O and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
8. It is a member of the chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioac ...
group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic ide ...
in the periodic table, a highly reactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
nonmetal, and an oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind
BIND () is a suite of software for interacting with the Domain Name System (DNS). Its most prominent component, named (pronounced ''name-dee'': , short for ''name daemon''), performs both of the main DNS server roles, acting as an authoritative ...
to form dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
* ...
, a colorless and odorless diatomic gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman.
Many major classes of organic molecule
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s in living organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and fungi ...
s contain oxygen atoms, such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, nucleic acids, carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
s, and fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple est ...
s, as do the major constituent inorganic compounds of animal shells, teeth, and bone. Most of the mass of living organisms is oxygen as a component of water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, the major constituent of lifeforms. Oxygen is continuously replenished in Earth's atmosphere by photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, which uses the energy of sunlight to produce oxygen from water and carbon dioxide. Oxygen is too chemically reactive to remain a free element in air without being continuously replenished by the photosynthetic action of living organisms. Another form ( allotrope) of oxygen, ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
(), strongly absorbs ultraviolet UVB
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
radiation and the high-altitude ozone layer helps protect the biosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
from ultraviolet radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
. However, ozone present at the surface is a byproduct of smog and thus a pollutant.
Oxygen was isolated by Michael Sendivogius
Michael Sendivogius (; pl, Michał Sędziwój; 2 February 1566 – 1636) was a Polish alchemist, philosopher, and medical doctor. A pioneer of chemistry, he developed ways of purification and creation of various acids, metals and other ch ...
before 1604, but it is commonly believed that the element was discovered independently by Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hyd ...
, in Uppsala
Uppsala (, or all ending in , ; archaically spelled ''Upsala'') is the county seat of Uppsala County and the fourth-largest city in Sweden, after Stockholm, Gothenburg, and Malmö. It had 177,074 inhabitants in 2019.
Located north of the c ...
, in 1773 or earlier, and Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
in Wiltshire
Wiltshire (; abbreviated Wilts) is a historic and ceremonial county in South West England with an area of . It is landlocked and borders the counties of Dorset to the southwest, Somerset to the west, Hampshire to the southeast, Gloucestershire ...
, in 1774. Priority is often given for Priestley because his work was published first. Priestley, however, called oxygen "dephlogisticated air", and did not recognize it as a chemical element. The name ''oxygen'' was coined in 1777 by Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794),
CNRS (
CNRS (
steel,
 Polish alchemist, philosopher, and
Polish alchemist, philosopher, and
 Lavoisier conducted the first adequate quantitative experiments on
Lavoisier conducted the first adequate quantitative experiments on
 John Dalton's original atomic hypothesis presumed that all elements were monatomic and that the atoms in compounds would normally have the simplest atomic ratios with respect to one another. For example, Dalton assumed that water's formula was HO, leading to the conclusion that the
John Dalton's original atomic hypothesis presumed that all elements were monatomic and that the atoms in compounds would normally have the simplest atomic ratios with respect to one another. For example, Dalton assumed that water's formula was HO, leading to the conclusion that the  In 1891 Scottish chemist
In 1891 Scottish chemist
 At standard temperature and pressure, oxygen is a colorless, odorless, and tasteless gas with the
At standard temperature and pressure, oxygen is a colorless, odorless, and tasteless gas with the
Google Books
accessed January 31, 2015. This combination of cancellations and σ and π overlaps results in dioxygen's double-bond character and reactivity, and a triplet electronic ground state. An electron configuration with two unpaired electrons, as is found in dioxygen orbitals (see the filled π* orbitals in the diagram) that are of equal energy—i.e., In the triplet form, molecules are
In the triplet form, molecules are
 The common allotrope of elemental oxygen on Earth is called
The common allotrope of elemental oxygen on Earth is called
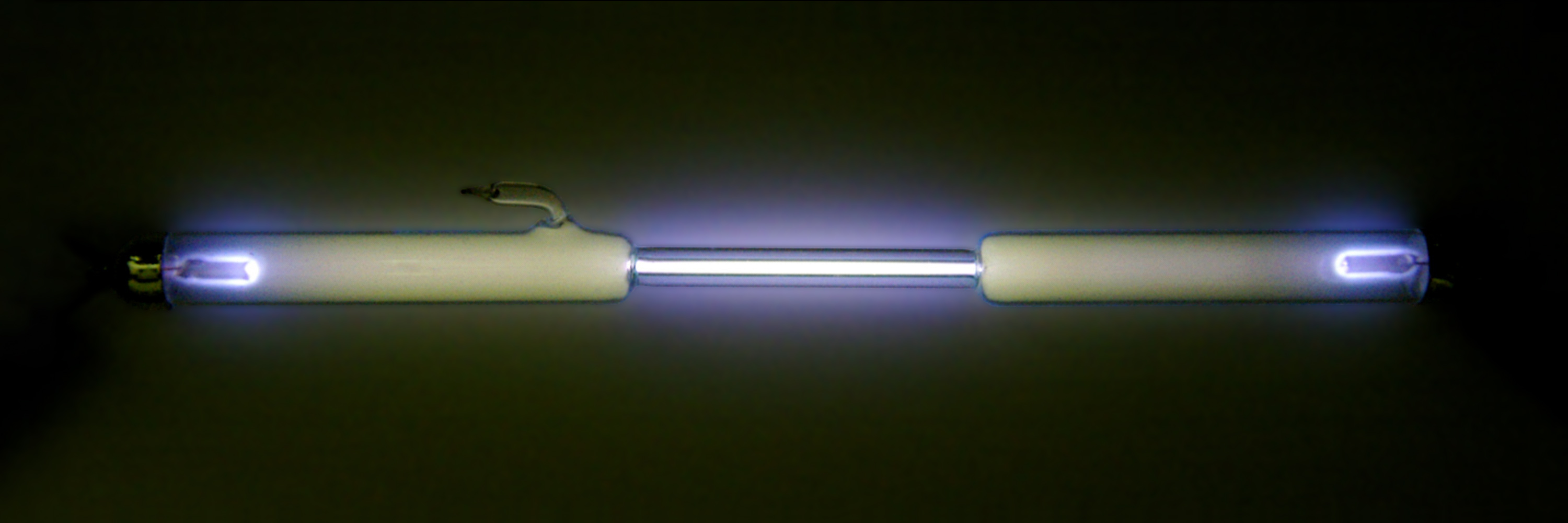 Oxygen dissolves more readily in water than in nitrogen, and in freshwater more readily than in seawater. Water in equilibrium with air contains approximately 1 molecule of dissolved for every 2 molecules of (1:2), compared with an atmospheric ratio of approximately 1:4. The solubility of oxygen in water is temperature-dependent, and about twice as much () dissolves at 0 °C than at 20 °C (). At 25 °C and of air, freshwater can dissolve about 6.04
Oxygen dissolves more readily in water than in nitrogen, and in freshwater more readily than in seawater. Water in equilibrium with air contains approximately 1 molecule of dissolved for every 2 molecules of (1:2), compared with an atmospheric ratio of approximately 1:4. The solubility of oxygen in water is temperature-dependent, and about twice as much () dissolves at 0 °C than at 20 °C (). At 25 °C and of air, freshwater can dissolve about 6.04
 Naturally occurring oxygen is composed of three stable
Naturally occurring oxygen is composed of three stable
 The unusually high concentration of oxygen gas on Earth is the result of the
The unusually high concentration of oxygen gas on Earth is the result of the

 In nature, free oxygen is produced by the light-driven splitting of water during oxygenic
In nature, free oxygen is produced by the light-driven splitting of water during oxygenic
 Free oxygen gas was almost nonexistent in
Free oxygen gas was almost nonexistent in
 One hundred million tonnes of are extracted from air for industrial uses annually by two primary methods. The most common method is fractional distillation of liquefied air, with
One hundred million tonnes of are extracted from air for industrial uses annually by two primary methods. The most common method is fractional distillation of liquefied air, with

 Uptake of from the air is the essential purpose of
Uptake of from the air is the essential purpose of
 An application of as a low-pressure
An application of as a low-pressure

 The
The
 Due to its
Due to its
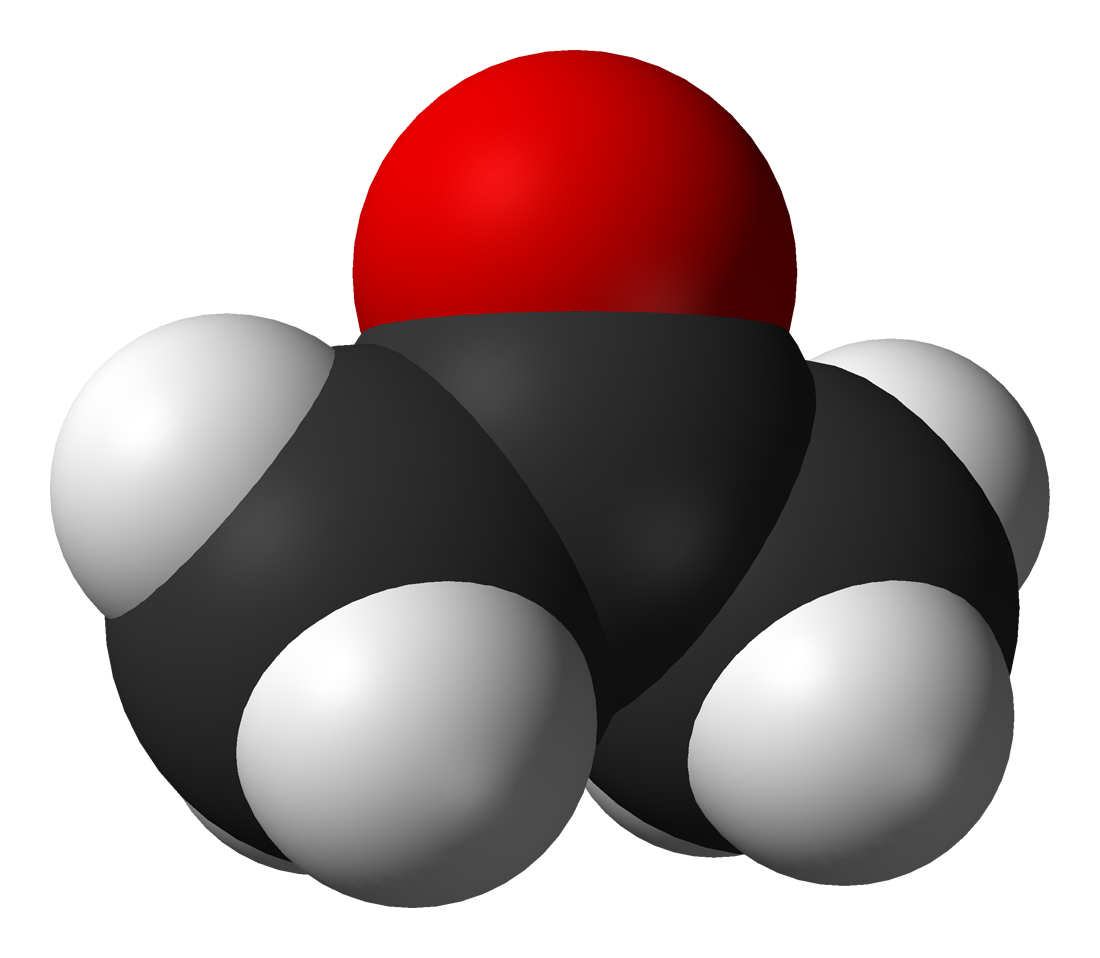 Among the most important classes of organic compounds that contain oxygen are (where "R" is an organic group): alcohols (R-OH);
Among the most important classes of organic compounds that contain oxygen are (where "R" is an organic group): alcohols (R-OH);
 Oxygen gas () can be
Oxygen gas () can be
 Highly concentrated sources of oxygen promote rapid combustion.
Highly concentrated sources of oxygen promote rapid combustion.
Oxygen
at ''The Periodic Table of Videos'' (University of Nottingham)
Oxidizing Agents > Oxygen
Roald Hoffmann article on "The Story of O"
*
Scripps Institute: Atmospheric Oxygen has been dropping for 20 years
{{featured article Oxygen, Chemical elements Diatomic nonmetals Reactive nonmetals Chalcogens Chemical substances for emergency medicine Breathing gases E-number additives Oxidizing agents
plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptab ...
s and textile
Textile is an umbrella term that includes various fiber-based materials, including fibers, yarns, filaments, threads, different fabric types, etc. At first, the word "textiles" only referred to woven fabrics. However, weaving is not the ...
s, brazing, welding and cutting of steels and other metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s, rocket propellant, oxygen therapy, and life support systems in aircraft
An aircraft is a vehicle that is able to fly by gaining support from the air. It counters the force of gravity by using either static lift or by using the dynamic lift of an airfoil, or in a few cases the downward thrust from jet engine ...
, submarines, spaceflight
Spaceflight (or space flight) is an application of astronautics to fly spacecraft into or through outer space, either with or without humans on board. Most spaceflight is uncrewed and conducted mainly with spacecraft such as satellites in o ...
and diving
Diving most often refers to:
* Diving (sport), the sport of jumping into deep water
* Underwater diving, human activity underwater for recreational or occupational purposes
Diving or Dive may also refer to:
Sports
* Dive (American football), a ...
.
History of study
Early experiments
One of the first known experiments on the relationship betweencombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combus ...
and air was conducted by the 2nd century BCE Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
writer on mechanics, Philo of Byzantium
Philo of Byzantium ( el, , ''Phílōn ho Byzántios'', ca. 280 BC – ca. 220 BC), also known as Philo Mechanicus, was a Greek engineer, physicist and writer on mechanics, who lived during the latter half of the 3rd century BC. Although he was f ...
. In his work ''Pneumatica'', Philo observed that inverting a vessel over a burning candle and surrounding the vessel's neck with water resulted in some water rising into the neck. Philo incorrectly surmised that parts of the air in the vessel were converted into the classical element fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames a ...
and thus were able to escape through pores in the glass. Many centuries later Leonardo da Vinci
Leonardo di ser Piero da Vinci (15 April 14522 May 1519) was an Italian polymath of the High Renaissance who was active as a painter, Drawing, draughtsman, engineer, scientist, theorist, sculptor, and architect. While his fame initially res ...
built on Philo's work by observing that a portion of air is consumed during combustion and respiration
Respiration may refer to:
Biology
* Cellular respiration, the process in which nutrients are converted into useful energy in a cell
** Anaerobic respiration, cellular respiration without oxygen
** Maintenance respiration, the amount of cellul ...
. Cook & Lauer 1968, p. 499.
In the late 17th century, Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
proved that air is necessary for combustion. English chemist John Mayow
John Mayow FRS (1641–1679) was a chemist, physician, and physiologist who is remembered today for conducting early research into respiration and the nature of air. Mayow worked in a field that is sometimes called pneumatic chemistry.
Lif ...
(1641–1679) refined this work by showing that fire requires only a part of air that he called ''spiritus nitroaereus''. In one experiment, he found that placing either a mouse or a lit candle in a closed container over water caused the water to rise and replace one-fourteenth of the air's volume before extinguishing the subjects. From this, he surmised that nitroaereus is consumed in both respiration and combustion.
Mayow observed that antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
increased in weight when heated, and inferred that the nitroaereus must have combined with it. He also thought that the lungs separate nitroaereus from air and pass it into the blood and that animal heat and muscle movement result from the reaction of nitroaereus with certain substances in the body. Accounts of these and other experiments and ideas were published in 1668 in his work ''Tractatus duo'' in the tract "De respiratione".
Phlogiston theory
Robert Hooke, Ole Borch, Mikhail Lomonosov, and Pierre Bayen all produced oxygen in experiments in the 17th and the 18th century but none of them recognized it as achemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
. Emsley 2001, p. 299 This may have been in part due to the prevalence of the philosophy of combustion and corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
called the ''phlogiston theory'', which was then the favored explanation of those processes.
Established in 1667 by the German alchemist J. J. Becher, and modified by the chemist Georg Ernst Stahl
Georg Ernst Stahl (22 October 1659 – 24 May 1734) was a German chemist, physician and philosopher. He was a supporter of vitalism, and until the late 18th century his works on phlogiston were accepted as an explanation for chemical processes.K ...
by 1731, phlogiston theory stated that all combustible materials were made of two parts. One part, called phlogiston, was given off when the substance containing it was burned, while the dephlogisticated part was thought to be its true form, or calx
Calx is a substance formed from an ore or mineral that has been heated.
Calx, especially of a metal, is now known as an oxide. According to the obsolete phlogiston theory, the calx was the true elemental substance, having lost its phlogiston in t ...
.
Highly combustible materials that leave little residue
Residue may refer to:
Chemistry and biology
* An amino acid, within a peptide chain
* Crop residue, materials left after agricultural processes
* Pesticide residue, refers to the pesticides that may remain on or in food after they are applied ...
, such as wood or coal, were thought to be made mostly of phlogiston; non-combustible substances that corrode, such as iron, contained very little. Air did not play a role in phlogiston theory, nor were any initial quantitative experiments conducted to test the idea; instead, it was based on observations of what happens when something burns, that most common objects appear to become lighter and seem to lose something in the process.
Discovery
 Polish alchemist, philosopher, and
Polish alchemist, philosopher, and physician
A physician (American English), medical practitioner (Commonwealth English), medical doctor, or simply doctor, is a health professional who practices medicine, which is concerned with promoting, maintaining or restoring health through th ...
Michael Sendivogius
Michael Sendivogius (; pl, Michał Sędziwój; 2 February 1566 – 1636) was a Polish alchemist, philosopher, and medical doctor. A pioneer of chemistry, he developed ways of purification and creation of various acids, metals and other ch ...
(Michał Sędziwój) in his work ''De Lapide Philosophorum Tractatus duodecim e naturae fonte et manuali experientia depromti'' (1604) described a substance contained in air, referring to it as 'cibus vitae' (food of life,) and according to Polish historian Roman Bugaj, this substance is identical with oxygen. Sendivogius, during his experiments performed between 1598 and 1604, properly recognized that the substance is equivalent to the gaseous byproduct released by the thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is re ...
of potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and nitr ...
. In Bugaj's view, the isolation of oxygen and the proper association of the substance to that part of air which is required for life, provides sufficient evidence for the discovery of oxygen by Sendivogius. This discovery of Sendivogius was however frequently denied by the generations of scientists and chemists which succeeded him.
It is also commonly claimed that oxygen was first discovered by Swedish pharmacist Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hyd ...
. He had produced oxygen gas by heating mercuric oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is v ...
(HgO) and various nitrates in 1771–72. Scheele called the gas "fire air" because it was then the only known agent
Agent may refer to:
Espionage, investigation, and law
*, spies or intelligence officers
* Law of agency, laws involving a person authorized to act on behalf of another
** Agent of record, a person with a contractual agreement with an insuranc ...
to support combustion. He wrote an account of this discovery in a manuscript titled ''Treatise on Air and Fire'', which he sent to his publisher in 1775. That document was published in 1777. Emsley 2001, p. 300
In the meantime, on August 1, 1774, an experiment conducted by the British clergyman Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
focused sunlight on mercuric oxide contained in a glass tube, which liberated a gas he named "dephlogisticated air". Cook & Lauer 1968, p. 500 He noted that candles burned brighter in the gas and that a mouse was more active and lived longer while breathing it. After breathing the gas himself, Priestley wrote: "The feeling of it to my lungs was not sensibly different from that of common air, but I fancied that my breast felt peculiarly light and easy for some time afterwards." Priestley published his findings in 1775 in a paper titled "An Account of Further Discoveries in Air", which was included in the second volume of his book titled ''Experiments and Observations on Different Kinds of Air
''Experiments and Observations on Different Kinds of Air'' (1774–86) is a six-volume work published by 18th-century British polymath Joseph Priestley which reports a series of his experiments on "airs" or gases, most notably his discovery of ...
''. Because he published his findings first, Priestley is usually given priority in the discovery.
The French chemist Antoine Laurent Lavoisier later claimed to have discovered the new substance independently. Priestley visited Lavoisier in October 1774 and told him about his experiment and how he liberated the new gas. Scheele had also dispatched a letter to Lavoisier on September 30, 1774, which described his discovery of the previously unknown substance, but Lavoisier never acknowledged receiving it. (A copy of the letter was found in Scheele's belongings after his death.)
Lavoisier's contribution
 Lavoisier conducted the first adequate quantitative experiments on
Lavoisier conducted the first adequate quantitative experiments on oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
and gave the first correct explanation of how combustion works. He used these and similar experiments, all started in 1774, to discredit the phlogiston theory and to prove that the substance discovered by Priestley and Scheele was a chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
.
In one experiment, Lavoisier observed that there was no overall increase in weight when tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
and air were heated in a closed container. He noted that air rushed in when he opened the container, which indicated that part of the trapped air had been consumed. He also noted that the tin had increased in weight and that increase was the same as the weight of the air that rushed back in. This and other experiments on combustion were documented in his book ''Sur la combustion en général'', which was published in 1777. In that work, he proved that air is a mixture of two gases; 'vital air', which is essential to combustion and respiration, and ''azote'' (Gk. ' "lifeless"), which did not support either. ''Azote'' later became ''nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
'' in English, although it has kept the earlier name in French and several other European languages.
Etymology
Lavoisier renamed 'vital air' to ''oxygène'' in 1777 from theGreek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
roots '' (oxys)'' ( acid, literally "sharp", from the taste of acids) and ''-γενής (-genēs)'' (producer, literally begetter), because he mistakenly believed that oxygen was a constituent of all acids. Chemists (such as Sir Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
in 1812) eventually determined that Lavoisier was wrong in this regard, but by then the name was too well established.
''Oxygen'' entered the English language despite opposition by English scientists and the fact that the Englishman Priestley had first isolated the gas and written about it. This is partly due to a poem praising the gas titled "Oxygen" in the popular book '' The Botanic Garden'' (1791) by Erasmus Darwin, grandfather of Charles Darwin
Charles Robert Darwin ( ; 12 February 1809 – 19 April 1882) was an English naturalist, geologist, and biologist, widely known for his contributions to evolutionary biology. His proposition that all species of life have descended ...
.
Later history
 John Dalton's original atomic hypothesis presumed that all elements were monatomic and that the atoms in compounds would normally have the simplest atomic ratios with respect to one another. For example, Dalton assumed that water's formula was HO, leading to the conclusion that the
John Dalton's original atomic hypothesis presumed that all elements were monatomic and that the atoms in compounds would normally have the simplest atomic ratios with respect to one another. For example, Dalton assumed that water's formula was HO, leading to the conclusion that the atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nb ...
of oxygen was 8 times that of hydrogen, instead of the modern value of about 16. In 1805, Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (, , ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen (with Alexander von Humboldt), for two laws ...
and Alexander von Humboldt
Friedrich Wilhelm Heinrich Alexander von Humboldt (14 September 17696 May 1859) was a German polymath, geographer, naturalist, explorer, and proponent of Romantic philosophy and science. He was the younger brother of the Prussian minister, ...
showed that water is formed of two volumes of hydrogen and one volume of oxygen; and by 1811 Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (, also , ; 9 August 17769 July 1856) was an Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volume ...
had arrived at the correct interpretation of water's composition, based on what is now called Avogadro's law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific c ...
and the diatomic elemental molecules in those gases.These results were mostly ignored until 1860. Part of this rejection was due to the belief that atoms of one element would have no chemical affinity In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom or compound to co ...
towards atoms of the same element, and part was due to apparent exceptions to Avogadro's law that were not explained until later in terms of dissociating molecules.
The first commercial method of producing oxygen was chemical, the so-called Brin process involving a reversible reaction of barium oxide
Barium oxide, also known as baria, is a white hygroscopic non-flammable compound with the formula BaO. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and if swallowed in larg ...
. It was invented in 1852 and commercialized in 1884, but was displaced by newer methods in early 20th century.
By the late 19th century scientists realized that air could be liquefied and its components isolated by compressing and cooling it. Using a cascade method, Swiss chemist and physicist Raoul Pierre Pictet evaporated
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humi ...
liquid sulfur dioxide in order to liquefy carbon dioxide, which in turn was evaporated to cool oxygen gas enough to liquefy it. He sent a telegram on December 22, 1877, to the French Academy of Sciences in Paris announcing his discovery of liquid oxygen
Liquid oxygen—abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries—is the liquid form of molecular oxygen. It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an app ...
. Just two days later, French physicist Louis Paul Cailletet
Louis-Paul Cailletet (21 September 1832 – 5 January 1913) was a French physicist and inventor.
Life and work
Cailletet was born in Châtillon-sur-Seine, Côte-d'Or. Educated in Paris, Cailletet returned to Châtillon to manage his fath ...
announced his own method of liquefying molecular oxygen. Only a few drops of the liquid were produced in each case and no meaningful analysis could be conducted. Oxygen was liquefied in a stable state for the first time on March 29, 1883, by Polish scientists from Jagiellonian University, Zygmunt Wróblewski Zygmunt, Zigmunt, Zigmund and spelling variations thereof are masculine given names and occasionally surnames. People so named include:
Given name Medieval period
* Sigismund I the Old (1467–1548), Zygmunt I Stary in Polish, King of Poland and Gr ...
and Karol Olszewski
Karol Stanisław Olszewski (29 January 1846 – 24 March 1915) was a Poles, Polish chemist, mathematician and physicist.
Biography
Olszewski was a graduate of Kazimierz Brodziński High School in Tarnów (I Liceum Ogólnokształcące im. Kazi ...
.
 In 1891 Scottish chemist
In 1891 Scottish chemist James Dewar
Sir James Dewar (20 September 1842 – 27 March 1923) was a British chemist and physicist. He is best known for his invention of the vacuum flask, which he used in conjunction with research into the liquefaction of gases. He also studied a ...
was able to produce enough liquid oxygen for study. Emsley 2001, p. 303 The first commercially viable process for producing liquid oxygen was independently developed in 1895 by German engineer Carl von Linde
Carl Paul Gottfried von Linde (11 June 1842 – 16 November 1934) was a German scientist, engineer, and businessman. He discovered a refrigeration cycle and invented the first industrial-scale air separation and gas liquefaction processes, whi ...
and British engineer William Hampson. Both men lowered the temperature of air until it liquefied and then distilled the component gases by boiling them off one at a time and capturing them separately. Later, in 1901, oxyacetylene welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as bra ...
was demonstrated for the first time by burning a mixture of acetylene and compressed . This method of welding and cutting metal later became common.
In 1923, the American scientist Robert H. Goddard
Robert Hutchings Goddard (October 5, 1882 – August 10, 1945) was an American engineer, professor, physicist, and inventor who is credited with creating and building the world's first liquid-fueled rocket. Goddard successfully laun ...
became the first person to develop a rocket engine
A rocket engine uses stored rocket propellants as the reaction mass for forming a high-speed propulsive jet of fluid, usually high-temperature gas. Rocket engines are reaction engines, producing thrust by ejecting mass rearward, in accorda ...
that burned liquid fuel; the engine used gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organi ...
for fuel and liquid oxygen as the oxidizer
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
. Goddard successfully flew a small liquid-fueled rocket 56 m at 97 km/h on March 16, 1926, in Auburn, Massachusetts
Auburn is a town in Worcester County, Massachusetts, United States. The population was 16,889 at the 2020 census.
History
The Auburn area was first settled in 1714 as of today outer parts of Worcester, Sutton, Leicester and Oxford, Massachus ...
, US.
In academic laboratories, oxygen can be prepared by heating together potassium chlorate mixed with a small proportion of manganese dioxide.
Oxygen levels in the atmosphere are trending slightly downward globally, possibly because of fossil-fuel burning.
Characteristics
Properties and molecular structure
molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
, referred to as dioxygen.
As ''dioxygen'', two oxygen atoms are chemically bound to each other. The bond can be variously described based on level of theory, but is reasonably and simply described as a covalent double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
that results from the filling of molecular orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
formed from the atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
s of the individual oxygen atoms, the filling of which results in a bond order of two. More specifically, the double bond is the result of sequential, low-to-high energy, or Aufbau, filling of orbitals, and the resulting cancellation of contributions from the 2s electrons, after sequential filling of the low σ and σ* orbitals; σ overlap of the two atomic 2p orbitals that lie along the O–O molecular axis and π overlap of two pairs of atomic 2p orbitals perpendicular to the O–O molecular axis, and then cancellation of contributions from the remaining two 2p electrons after their partial filling of the π* orbitals.Jack Barrett, 2002, "Atomic Structure and Periodicity", (Basic concepts in chemistry, Vol. 9 of Tutorial chemistry texts), Cambridge, UK: Royal Society of Chemistry, p. 153, . SeGoogle Books
accessed January 31, 2015. This combination of cancellations and σ and π overlaps results in dioxygen's double-bond character and reactivity, and a triplet electronic ground state. An electron configuration with two unpaired electrons, as is found in dioxygen orbitals (see the filled π* orbitals in the diagram) that are of equal energy—i.e.,
degenerate
Degeneracy, degenerate, or degeneration may refer to:
Arts and entertainment
* Degenerate (album), ''Degenerate'' (album), a 2010 album by the British band Trigger the Bloodshed
* Degenerate art, a term adopted in the 1920s by the Nazi Party i ...
—is a configuration termed a spin triplet
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical r ...
state. Hence, the ground state of the molecule is referred to as triplet oxygen
Triplet oxygen, 3O2, refers to the ''S'' = 1 electronic ground state of molecular oxygen (dioxygen). It is the most stable and common allotrope of oxygen. Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unus ...
.An orbital is a concept from quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistr ...
that models an electron as a wave-like particle that has a spatial distribution about an atom or molecule. The highest-energy, partially filled orbitals are antibonding, and so their filling weakens the bond order from three to two. Because of its unpaired electrons, triplet oxygen reacts only slowly with most organic molecules, which have paired electron spins; this prevents spontaneous combustion.
 In the triplet form, molecules are
In the triplet form, molecules are paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. That is, they impart magnetic character to oxygen when it is in the presence of a magnetic field, because of the spin magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagne ...
s of the unpaired electrons in the molecule, and the negative exchange energy between neighboring molecules. Liquid oxygen is so magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nicke ...
ic that, in laboratory demonstrations, a bridge of liquid oxygen may be supported against its own weight between the poles of a powerful magnet.
Singlet oxygen is a name given to several higher-energy species of molecular in which all the electron spins are paired. It is much more reactive with common organic molecules than is normal (triplet) molecular oxygen. In nature, singlet oxygen is commonly formed from water during photosynthesis, using the energy of sunlight. It is also produced in the troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
by the photolysis of ozone by light of short wavelength and by the immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint ...
as a source of active oxygen. Carotenoids in photosynthetic organisms (and possibly animals) play a major role in absorbing energy from singlet oxygen and converting it to the unexcited ground state before it can cause harm to tissues.
Allotropes
 The common allotrope of elemental oxygen on Earth is called
The common allotrope of elemental oxygen on Earth is called dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
* ...
, , the major part of the Earth's atmospheric oxygen (see Occurrence). O2 has a bond length of 121 pm and a bond energy of 498 kJ/mol. O2 is used by complex forms of life, such as animals, in cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
. Other aspects of are covered in the remainder of this article.
Trioxygen () is usually known as ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
and is a very reactive allotrope of oxygen that is damaging to lung tissue. Ozone is produced in the upper atmosphere Upper atmosphere is a collective term that refers to various layers of the atmosphere of the Earth above the troposphere and corresponding regions of the atmospheres of other planets, and includes:
* The mesosphere, which on Earth lies between th ...
when combines with atomic oxygen made by the splitting of by ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
(UV) radiation. Since ozone absorbs strongly in the UV region of the spectrum
A spectrum (plural ''spectra'' or ''spectrums'') is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors ...
, the ozone layer of the upper atmosphere functions as a protective radiation shield for the planet. Near the Earth's surface, it is a pollutant
A pollutant or novel entity is a substance or energy introduced into the environment that has undesired effects, or adversely affects the usefulness of a resource. These can be both naturally forming (i.e. minerals or extracted compounds like o ...
formed as a by-product of automobile exhaust. At low earth orbit
A low Earth orbit (LEO) is an orbit around Earth with a period of 128 minutes or less (making at least 11.25 orbits per day) and an eccentricity less than 0.25. Most of the artificial objects in outer space are in LEO, with an altitude never mor ...
altitudes, sufficient atomic oxygen is present to cause corrosion of spacecraft.
The metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
molecule tetraoxygen
The tetraoxygen molecule (O4), also called oxozone, is an allotrope of oxygen consisting of four oxygen atoms.
History
Tetraoxygen was first predicted in 1924 by Gilbert N. Lewis, who proposed it as an explanation for the failure of liquid oxyge ...
() was discovered in 2001, and was assumed to exist in one of the six phases of solid oxygen
Solid oxygen forms at normal atmospheric pressure at a temperature below 54.36 K (−218.79 °C, −361.82 °F). Solid oxygen O2, like liquid oxygen, is a clear substance with a light sky-blue color caused by absorption in the red part ...
. It was proven in 2006 that this phase, created by pressurizing to 20 GPa
Grading in education is the process of applying standardized measurements for varying levels of achievements in a course. Grades can be assigned as letters (usually A through F), as a range (for example, 1 to 6), as a percentage, or as a numbe ...
, is in fact a rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a three-dimensional figure with six faces which are rhombi. It is a special case of a parallelepiped where all edges are the same length. It can be us ...
cluster
may refer to:
Science and technology Astronomy
* Cluster (spacecraft), constellation of four European Space Agency spacecraft
* Asteroid cluster, a small asteroid family
* Cluster II (spacecraft), a European Space Agency mission to study t ...
. This cluster has the potential to be a much more powerful oxidizer
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
than either or and may therefore be used in rocket fuel
Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical ...
. A metallic phase was discovered in 1990 when solid oxygen is subjected to a pressure of above 96 GPa and it was shown in 1998 that at very low temperatures, this phase becomes superconducting
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
.
Physical properties
 Oxygen dissolves more readily in water than in nitrogen, and in freshwater more readily than in seawater. Water in equilibrium with air contains approximately 1 molecule of dissolved for every 2 molecules of (1:2), compared with an atmospheric ratio of approximately 1:4. The solubility of oxygen in water is temperature-dependent, and about twice as much () dissolves at 0 °C than at 20 °C (). At 25 °C and of air, freshwater can dissolve about 6.04
Oxygen dissolves more readily in water than in nitrogen, and in freshwater more readily than in seawater. Water in equilibrium with air contains approximately 1 molecule of dissolved for every 2 molecules of (1:2), compared with an atmospheric ratio of approximately 1:4. The solubility of oxygen in water is temperature-dependent, and about twice as much () dissolves at 0 °C than at 20 °C (). At 25 °C and of air, freshwater can dissolve about 6.04 milliliters
The litre (international spelling) or liter (American English spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metre (m3) ...
(mL) of oxygen per liter, and seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appr ...
contains about 4.95 mL per liter. At 5 °C the solubility increases to 9.0 mL (50% more than at 25 °C) per liter for freshwater and 7.2 mL (45% more) per liter for sea water.
Oxygen condenses at 90.20 K (−182.95 °C, −297.31 °F) and freezes at 54.36 K (−218.79 °C, −361.82 °F). Both liquid and solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural ...
are clear substances with a light sky-blue color caused by absorption in the red (in contrast with the blue color of the sky, which is due to Rayleigh scattering
Rayleigh scattering ( ), named after the 19th-century British physicist Lord Rayleigh (John William Strutt), is the predominantly elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of th ...
of blue light). High-purity liquid is usually obtained by the fractional distillation of liquefied air. Liquid oxygen may also be condensed from air using liquid nitrogen as a coolant.
Liquid oxygen is a highly reactive substance and must be segregated from combustible materials.
The spectroscopy of molecular oxygen is associated with the atmospheric processes of aurora
An aurora (plural: auroras or aurorae), also commonly known as the polar lights, is a natural light display in Earth's sky, predominantly seen in high-latitude regions (around the Arctic and Antarctic). Auroras display dynamic patterns of bri ...
and airglow
Airglow (also called nightglow) is a faint emission of light by a planetary atmosphere. In the case of Earth's atmosphere, this optical phenomenon causes the night sky never to be completely dark, even after the effects of starlight and diffu ...
. The absorption in the Herzberg continuum Herzberg is German for "heart mountain" and may refer to:
Places in Germany
* Herzberg am Harz, a town in the Osterode district, Lower Saxony
*Herzberg, Brandenburg, a town in the Elbe-Elster district, Brandenburg
*Herzberg, Ostprignitz-Ruppin, a t ...
and Schumann–Runge bands in the ultraviolet produces atomic oxygen that is important in the chemistry of the middle atmosphere. Excited-state singlet molecular oxygen is responsible for red chemiluminescence in solution.
Table of thermal and physical properties of oxygen (O2) at atmospheric pressure:
Isotopes and stellar origin
isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
s, 16O, 17O, and 18O, with 16O being the most abundant (99.762% natural abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomi ...
).
Most 16O is synthesized at the end of the helium fusion
The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.
Triple-alpha process in stars
Helium accumulates in the cores of stars as a result of the proton–pro ...
process in massive stars but some is made in the neon burning process
The neon-burning process is a set of nuclear fusion reactions that take place in evolved massive stars with at least 8 Solar masses. Neon burning requires high temperatures and densities (around 1.2×109 K or 100 keV and 4×109 kg/m3).
At such h ...
. 17O is primarily made by the burning of hydrogen into helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
during the CNO cycle
The CNO cycle (for carbon–nitrogen–oxygen; sometimes called Bethe–Weizsäcker cycle after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker) is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, ...
, making it a common isotope in the hydrogen burning zones of stars. Most 18O is produced when 14N (made abundant from CNO burning) captures a 4He nucleus, making 18O common in the helium-rich zones of evolved, massive stars.
Fourteen radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s have been characterized. The most stable are 15O with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 122.24 seconds and 14O with a half-life of 70.606 seconds. All of the remaining radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
isotopes have half-lives that are less than 27 s and the majority of these have half-lives that are less than 83 milliseconds. The most common decay mode of the isotopes lighter than 16O is β+ decay to yield nitrogen, and the most common mode for the isotopes heavier than 18O is beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
to yield fluorine.
Occurrence
Oxygen is the most abundant chemical element by mass in the Earth'sbiosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
, air, sea and land. Oxygen is the third most abundant chemical element in the universe, after hydrogen and helium. Emsley 2001, p. 297 About 0.9% of the Sun
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
's mass is oxygen. Oxygen constitutes 49.2% of the Earth's crust by mass as part of oxide compounds such as silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
and is the most abundant element by mass in the Earth's crust. It is also the major component of the world's oceans (88.8% by mass). Oxygen gas is the second most common component of the Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
, taking up 20.8% of its volume and 23.1% of its mass (some 1015 tonnes). Emsley 2001, p. 298Figures given are for values up to above the surface Earth is unusual among the planets of the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
in having such a high concentration of oxygen gas in its atmosphere: Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury. In the English language, Mars is named for the Roman god of war. Mars is a terrestrial planet with a thin at ...
(with 0.1% by volume) and Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never f ...
have much less. The surrounding those planets is produced solely by the action of ultraviolet radiation on oxygen-containing molecules such as carbon dioxide.
 The unusually high concentration of oxygen gas on Earth is the result of the
The unusually high concentration of oxygen gas on Earth is the result of the oxygen cycle
Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as wel ...
. This biogeochemical cycle describes the movement of oxygen within and between its three main reservoirs on Earth: the atmosphere, the biosphere, and the lithosphere. The main driving factor of the oxygen cycle is photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, which is responsible for modern Earth's atmosphere. Photosynthesis releases oxygen into the atmosphere, while respiration
Respiration may refer to:
Biology
* Cellular respiration, the process in which nutrients are converted into useful energy in a cell
** Anaerobic respiration, cellular respiration without oxygen
** Maintenance respiration, the amount of cellul ...
, decay
Decay may refer to:
Science and technology
* Bit decay, in computing
* Software decay, in computing
* Distance decay, in geography
* Decay time (fall time), in electronics
Biology
* Decomposition of organic matter
* Tooth decay (dental caries ...
, and combustion remove it from the atmosphere. In the present equilibrium, production and consumption occur at the same rate.
Free oxygen also occurs in solution in the world's water bodies. The increased solubility of at lower temperatures (see Physical properties) has important implications for ocean life, as polar oceans support a much higher density of life due to their higher oxygen content. Water polluted with plant nutrients such as nitrates or phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
s may stimulate growth of algae by a process called eutrophication
Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytopla ...
and the decay of these organisms and other biomaterials may reduce the content in eutrophic water bodies. Scientists assess this aspect of water quality by measuring the water's biochemical oxygen demand
Biochemical oxygen demand (BOD) is the amount of dissolved oxygen (DO) needed (i.e. demanded) by aerobic biological organisms to break down organic material present in a given water sample at a certain temperature over a specific time period. ...
, or the amount of needed to restore it to a normal concentration. Emsley 2001, p. 301
Analysis

Paleoclimatologists
Paleoclimatology ( British spelling, palaeoclimatology) is the study of climates for which direct measurements were not taken. As instrumental records only span a tiny part of Earth's history, the reconstruction of ancient climate is important t ...
measure the ratio of oxygen-18 and oxygen-16 in the shells and skeletons of marine organisms to determine the climate millions of years ago (see oxygen isotope ratio cycle
Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite in ocean core sampl ...
). Seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appr ...
molecules that contain the lighter isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
, oxygen-16, evaporate at a slightly faster rate than water molecules containing the 12% heavier oxygen-18, and this disparity increases at lower temperatures. Emsley 2001, p. 304 During periods of lower global temperatures, snow and rain from that evaporated water tends to be higher in oxygen-16, and the seawater left behind tends to be higher in oxygen-18. Marine organisms then incorporate more oxygen-18 into their skeletons and shells than they would in a warmer climate. Paleoclimatologists also directly measure this ratio in the water molecules of ice core samples as old as hundreds of thousands of years.
Planetary geologists have measured the relative quantities of oxygen isotopes in samples from the Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surfa ...
, the Moon
The Moon is Earth's only natural satellite. It is the fifth largest satellite in the Solar System and the largest and most massive relative to its parent planet, with a diameter about one-quarter that of Earth (comparable to the width of ...
, Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury. In the English language, Mars is named for the Roman god of war. Mars is a terrestrial planet with a thin at ...
, and meteorites, but were long unable to obtain reference values for the isotope ratios in the Sun
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
, believed to be the same as those of the primordial solar nebula. Analysis of a silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
wafer exposed to the solar wind
The solar wind is a stream of charged particles released from the upper atmosphere of the Sun, called the corona. This plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the sol ...
in space and returned by the crashed Genesis spacecraft has shown that the Sun has a higher proportion of oxygen-16 than does the Earth. The measurement implies that an unknown process depleted oxygen-16 from the Sun's disk of protoplanetary material prior to the coalescence of dust grains that formed the Earth.
Oxygen presents two spectrophotometric absorption bands peaking at the wavelengths 687 and 760 nm. Some remote sensing
Remote sensing is the acquisition of information about an object or phenomenon without making physical contact with the object, in contrast to in situ or on-site observation. The term is applied especially to acquiring information about Eart ...
scientists have proposed using the measurement of the radiance coming from vegetation canopies in those bands to characterize plant health status from a satellite
A satellite or artificial satellite is an object intentionally placed into orbit in outer space. Except for passive satellites, most satellites have an electricity generation system for equipment on board, such as solar panels or radioi ...
platform. This approach exploits the fact that in those bands it is possible to discriminate the vegetation's reflectance
The reflectance of the surface of a material is its effectiveness in reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic ...
from its fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
, which is much weaker. The measurement is technically difficult owing to the low signal-to-noise ratio and the physical structure of vegetation; but it has been proposed as a possible method of monitoring the carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major componen ...
from satellites on a global scale.
Biological production and role of O2
Photosynthesis and respiration
photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
. According to some estimates, green algae and cyanobacteria in marine environments provide about 70% of the free oxygen produced on Earth, and the rest is produced by terrestrial plants. Other estimates of the oceanic contribution to atmospheric oxygen are higher, while some estimates are lower, suggesting oceans produce ~45% of Earth's atmospheric oxygen each year.
A simplified overall formula for photosynthesis is
: 6 + 6 + photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
s → + 6
or simply
: carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
+ water + sunlight → glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
+ dioxygen
Photolytic oxygen evolution
Oxygen evolution is the process of generating molecular oxygen (O2) by a chemical reaction, usually from water. Oxygen evolution from water is effected by oxygenic photosynthesis, electrolysis of water, and thermal decomposition of various oxides. ...
occurs in the thylakoid membrane
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thyla ...
s of photosynthetic organisms and requires the energy of four photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
s.Thylakoid membranes are part of chloroplasts in algae and plants while they simply are one of many membrane structures in cyanobacteria. In fact, chloroplasts are thought to have evolved from cyanobacteria that were once symbiotic partners with the progenitors of plants and algae. Many steps are involved, but the result is the formation of a proton gradient across the thylakoid membrane, which is used to synthesize adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms o ...
(ATP) via photophosphorylation In the process of photosynthesis, the phosphorylation of ADP to form ATP using the energy of sunlight is called photophosphorylation. Cyclic photophosphorylation occurs in both aerobic and anaerobic conditions, driven by the main primary source of ...
. Raven 2005, 115–27 The remaining (after production of the water molecule) is released into the atmosphere.Water oxidation is catalyzed by a manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
-containing enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
complex known as the oxygen evolving complex
The oxygen-evolving complex (OEC), also known as the water-splitting complex, is the portion of photosystem II where photo-oxidation of water occurs during the light reactions of photosynthesis. The OEC is surrounded by four core protein subunits ...
(OEC) or water-splitting complex found associated with the lumenal side of thylakoid membranes. Manganese is an important cofactor, and calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
and chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
are also required for the reaction to occur. (Raven 2005)
Oxygen is used in mitochondria in the generation of ATP during oxidative phosphorylation. The reaction for aerobic respiration is essentially the reverse of photosynthesis and is simplified as
: + 6 → 6 + 6 + 2880 kJ/mol
In vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () (chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, with c ...
s, diffuses through membranes in the lungs and into red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s. Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
binds , changing color from bluish red to bright red ( is released from another part of hemoglobin through the Bohr effect). Other animals use hemocyanin
Hemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2) ...
(molluscs
Mollusca is the second-largest phylum of invertebrate animals after the Arthropoda, the members of which are known as molluscs or mollusks (). Around 85,000 extant species of molluscs are recognized. The number of fossil species is estim ...
and some arthropod
Arthropods (, (gen. ποδός)) are invertebrate animals with an exoskeleton, a segmented body, and paired jointed appendages. Arthropods form the phylum Arthropoda. They are distinguished by their jointed limbs and cuticle made of chiti ...
s) or hemerythrin
Hemerythrin (also spelled haemerythrin; grc, αἷμα, haîma, blood, grc, ἐρυθρός, erythrós, red) is an oligomeric protein responsible for oxygen (O2) transport in the marine invertebrate phyla of sipunculids, priapulids, brachiop ...
(spider
Spiders ( order Araneae) are air-breathing arthropods that have eight legs, chelicerae with fangs generally able to inject venom, and spinnerets that extrude silk. They are the largest order of arachnids and rank seventh in total species ...
s and lobsters). A liter of blood can dissolve 200 cm3 of .
Until the discovery of anaerobic
Anaerobic means "living, active, occurring, or existing in the absence of free oxygen", as opposed to aerobic which means "living, active, or occurring only in the presence of oxygen." Anaerobic may also refer to:
* Anaerobic adhesive, a bonding a ...
metazoa
Animals are multicellular, eukaryotic organisms in the biological kingdom Animalia. With few exceptions, animals consume organic material, breathe oxygen, are able to move, can reproduce sexually, and go through an ontogenetic stage in ...
, oxygen was thought to be a requirement for all complex life.
Reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
, such as superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
ion () and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
(), are reactive by-products of oxygen use in organisms. Parts of the immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint ...
of higher organisms create peroxide, superoxide, and singlet oxygen to destroy invading microbes. Reactive oxygen species also play an important role in the hypersensitive response
Hypersensitive response (HR) is a mechanism used by plants to prevent the spread of infection by microbial pathogens. HR is characterized by the rapid death of cells in the local region surrounding an infection and it serves to restrict the growt ...
of plants against pathogen attack. Oxygen is damaging to obligately anaerobic organisms, which were the dominant form of early life
Early may refer to:
History
* The beginning or oldest part of a defined historical period, as opposed to middle or late periods, e.g.:
** Early Christianity
** Early modern Europe
Places in the United States
* Early, Iowa
* Early, Texas
* Early ...
on Earth until began to accumulate in the atmosphere about 2.5 billion years ago during the Great Oxygenation Event, about a billion years after the first appearance of these organisms.
An adult human at rest inhales 1.8 to 2.4 grams of oxygen per minute. This amounts to more than 6 billion tonnes of oxygen inhaled by humanity per year.(1.8 grams/min/person)×(60 min/h)×(24 h/day)×(365 days/year)×(6.6 billion people)/1,000,000 g/t=6.24 billion tonnes
Living organisms
The free oxygen partial pressure in the body of a living vertebrate organism is highest in therespiratory system
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies ...
, and decreases along any arterial system, peripheral tissues, and venous system
Veins are blood vessels in humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are the pulmonary and umbilical veins, both of which carry oxygenated ...
, respectively. Partial pressure is the pressure that oxygen would have if it alone occupied the volume.
Build-up in the atmosphere
Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
before photosynthetic archaea and bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
evolved, probably about 3.5 billion years ago. Free oxygen first appeared in significant quantities during the Paleoproterozoic
The Paleoproterozoic Era (;, also spelled Palaeoproterozoic), spanning the time period from (2.5–1.6 Ga), is the first of the three sub-divisions ( eras) of the Proterozoic Eon. The Paleoproterozoic is also the longest era of the Earth's ...
eon (between 3.0 and 2.3 billion years ago). Even if there was much dissolved iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
in the oceans when oxygenic photosynthesis was getting more common, it appears the banded iron formation
Banded iron formations (also known as banded ironstone formations or BIFs) are distinctive units of sedimentary rock consisting of alternating layers of iron oxides and iron-poor chert. They can be up to several hundred meters in thickness ...
s were created by anoxyenic or micro-aerophilic iron-oxidizing bacteria which dominated the deeper areas of the photic zone, while oxygen-producing cyanobacteria covered the shallows. Free oxygen began to outgas
Outgassing (sometimes called offgassing, particularly when in reference to indoor air quality) is the release of a gas that was dissolved, trapped, frozen, or absorbed in some material. Outgassing can include sublimation and evaporation (which ...
from the oceans 3–2.7 billion years ago, reaching 10% of its present level around 1.7 billion years ago.
The presence of large amounts of dissolved and free oxygen in the oceans and atmosphere may have driven most of the extant anaerobic organisms to extinction
Extinction is the termination of a kind of organism or of a group of kinds (taxon), usually a species. The moment of extinction is generally considered to be the death of the last individual of the species, although the capacity to breed and ...
during the Great Oxygenation Event (''oxygen catastrophe'') about 2.4 billion years ago. Cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
using enables aerobic organism
Aerobic means "requiring air," in which "air" usually means oxygen.
Aerobic may also refer to
* Aerobic exercise, prolonged exercise of moderate intensity
* Aerobics
Aerobics is a form of physical exercise that combines rhythmic aerobic exe ...
s to produce much more ATP than anaerobic organisms. Cellular respiration of occurs in all eukaryotes, including all complex multicellular organisms such as plants and animals.
Since the beginning of the Cambrian period 540 million years ago, atmospheric levels have fluctuated between 15% and 30% by volume. Towards the end of the Carboniferous period (about 300 million years ago) atmospheric levels reached a maximum of 35% by volume, which may have contributed to the large size of insects and amphibians at this time.
Variations in atmospheric oxygen concentration have shaped past climates. When oxygen declined, atmospheric density dropped, which in turn increased surface evaporation, causing precipitation increases and warmer temperatures.
At the current rate of photosynthesis it would take about 2,000 years to regenerate the entire in the present atmosphere.
Extraterrestrial free oxygen
In the field of astrobiology and in the search for extraterrestrial life oxygen is a strong biosignature. That said it might not be a definite biosignature, being possibly produced abiotically on celestial bodies with processes and conditions (such as a peculiarhydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to change in shape. This ...
) which allow free oxygen, like with Europa's and Ganymede's thin
oxygen atmospheres.
Industrial production
distilling
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating ...
as a vapor while is left as a liquid.
The other primary method of producing is passing a stream of clean, dry air through one bed of a pair of identical zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These p ...
molecular sieves, which absorbs the nitrogen and delivers a gas stream that is 90% to 93% . Simultaneously, nitrogen gas is released from the other nitrogen-saturated zeolite bed, by reducing the chamber operating pressure and diverting part of the oxygen gas from the producer bed through it, in the reverse direction of flow. After a set cycle time the operation of the two beds is interchanged, thereby allowing for a continuous supply of gaseous oxygen to be pumped through a pipeline. This is known as pressure swing adsorption
Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases (typically air) under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at ne ...
. Oxygen gas is increasingly obtained by these non- cryogenic technologies (see also the related vacuum swing adsorption
Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases (typically air) under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at nea ...
).
Oxygen gas can also be produced through electrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
into molecular oxygen and hydrogen. DC electricity must be used: if AC is used, the gases in each limb consist of hydrogen and oxygen in the explosive ratio 2:1. A similar method is the electrocatalytic evolution from oxides and oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce ...
s. Chemical catalysts can be used as well, such as in chemical oxygen generator
A chemical oxygen generator is a device that releases oxygen via a chemical reaction. The oxygen source is usually an inorganic superoxide, chlorate, or perchlorate; ozonides are a promising group of oxygen sources. The generators are usually ig ...
s or oxygen candles that are used as part of the life-support equipment on submarines, and are still part of standard equipment on commercial airliners in case of depressurization emergencies. Another air separation method is forcing air to dissolve through ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain ...
membranes based on zirconium dioxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabi ...
by either high pressure or an electric current, to produce nearly pure gas.
Storage

Oxygen storage Methods of oxygen storage for subsequent use span many approaches, including high pressures in oxygen tanks, cryogenics, oxygen-rich compounds and reaction mixtures, and chemical compounds that reversibly release oxygen upon heating or pressure cha ...
methods include high-pressure oxygen tanks, cryogenics and chemical compounds. For reasons of economy, oxygen is often transported in bulk as a liquid in specially insulated tankers, since one liter of liquefied oxygen is equivalent to 840 liters of gaseous oxygen at atmospheric pressure and . Such tankers are used to refill bulk liquid-oxygen storage containers, which stand outside hospitals and other institutions that need large volumes of pure oxygen gas. Liquid oxygen is passed through heat exchanger
A heat exchanger is a system used to transfer heat between a source and a working fluid. Heat exchangers are used in both cooling and heating processes. The fluids may be separated by a solid wall to prevent mixing or they may be in direct conta ...
s, which convert the cryogenic liquid into gas before it enters the building. Oxygen is also stored and shipped in smaller cylinders containing the compressed gas; a form that is useful in certain portable medical applications and oxy-fuel welding and cutting
Principle of burn cutting
Oxy-fuel welding (commonly called oxyacetylene welding, oxy welding, or gas welding in the United States) and oxy-fuel cutting are processes that use fuel gases (or liquid fuels such as gasoline or petrol, diesel, ...
.
Applications
Medical
 Uptake of from the air is the essential purpose of
Uptake of from the air is the essential purpose of respiration
Respiration may refer to:
Biology
* Cellular respiration, the process in which nutrients are converted into useful energy in a cell
** Anaerobic respiration, cellular respiration without oxygen
** Maintenance respiration, the amount of cellul ...
, so oxygen supplementation is used in medicine
Medicine is the science and practice of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care pr ...
. Treatment not only increases oxygen levels in the patient's blood, but has the secondary effect of decreasing resistance to blood flow in many types of diseased lungs, easing work load on the heart. Oxygen therapy is used to treat emphysema, pneumonia
Pneumonia is an inflammatory condition of the lung primarily affecting the small air sacs known as alveoli. Symptoms typically include some combination of productive or dry cough, chest pain, fever, and difficulty breathing. The severi ...
, some heart disorders (congestive heart failure
Heart failure (HF), also known as congestive heart failure (CHF), is a syndrome, a group of signs and symptoms caused by an impairment of the heart's blood pumping function. Symptoms typically include shortness of breath, excessive fatigue, ...
), some disorders that cause increased pulmonary artery pressure
A pulmonary artery is an artery in the pulmonary circulation that carries deoxygenated blood from the right side of the heart to the lungs. The largest pulmonary artery is the ''main pulmonary artery'' or ''pulmonary trunk'' from the heart, and ...
, and any disease
A disease is a particular abnormal condition that negatively affects the structure or function of all or part of an organism, and that is not immediately due to any external injury. Diseases are often known to be medical conditions that a ...
that impairs the body's ability to take up and use gaseous oxygen. Cook & Lauer 1968, p. 510
Treatments are flexible enough to be used in hospitals, the patient's home, or increasingly by portable devices. Oxygen tent
An oxygen tent consists of a canopy placed over the head and shoulders, or over the entire body of a patient to provide oxygen at a higher level than normal. Some devices cover only a part of the face. Oxygen tents are sometimes confused with a ...
s were once commonly used in oxygen supplementation, but have since been replaced mostly by the use of oxygen masks or nasal cannula
The nasal cannula (NC) is a device used to deliver supplemental oxygen or increased airflow to a patient or person in need of respiratory help. This device consists of a lightweight tube which on one end splits into two prongs which are place ...
s.
Hyperbaric
Hyperbaric medicine is medical treatment in which an ambient pressure greater than sea level atmospheric pressure is a necessary component. The treatment comprises hyperbaric oxygen therapy (HBOT), the medical use of oxygen at an ambient pressure ...
(high-pressure) medicine uses special oxygen chambers to increase the partial pressure of around the patient and, when needed, the medical staff. Carbon monoxide poisoning
Carbon monoxide poisoning typically occurs from breathing in carbon monoxide (CO) at excessive levels. Symptoms are often described as " flu-like" and commonly include headache, dizziness, weakness, vomiting, chest pain, and confusion. Large ...
, gas gangrene
Gas gangrene (also known as clostridial myonecrosis and myonecrosis) is a bacterial infection that produces tissue gas in gangrene. This deadly form of gangrene usually is caused by '' Clostridium perfringens'' bacteria. About 1,000 cases of gas ...
, and decompression sickness
Decompression sickness (abbreviated DCS; also called divers' disease, the bends, aerobullosis, and caisson disease) is a medical condition caused by dissolved gases emerging from solution as bubbles inside the body tissues during decompressio ...
(the 'bends') are sometimes addressed with this therapy. Increased concentration in the lungs helps to displace carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
from the heme group of hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
. Oxygen gas is poisonous to the anaerobic bacteria
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenat ...
that cause gas gangrene, so increasing its partial pressure helps kill them. Decompression sickness occurs in divers who decompress too quickly after a dive, resulting in bubbles of inert gas, mostly nitrogen and helium, forming in the blood. Increasing the pressure of as soon as possible helps to redissolve the bubbles back into the blood so that these excess gasses can be exhaled naturally through the lungs. Normobaric oxygen administration at the highest available concentration is frequently used as first aid for any diving injury that may involve inert gas bubble formation in the tissues. There is epidemiological support for its use from a statistical study of cases recorded in a long term database.
Life support and recreational use
 An application of as a low-pressure
An application of as a low-pressure breathing gas
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed ...
is in modern space suit
A space suit or spacesuit is a garment worn to keep a human alive in the harsh environment of outer space, vacuum and temperature extremes. Space suits are often worn inside spacecraft as a safety precaution in case of loss of cabin pressure, ...
s, which surround their occupant's body with the breathing gas. These devices use nearly pure oxygen at about one-third normal pressure, resulting in a normal blood partial pressure of . This trade-off of higher oxygen concentration for lower pressure is needed to maintain suit flexibility.
Scuba
Scuba may refer to:
* Scuba diving
** Scuba set, the equipment used for scuba (Self-Contained Underwater Breathing Apparatus) diving
* Scuba, an in-memory database developed by Facebook
* Submillimetre Common-User Bolometer Array, either of two in ...
and surface-supplied underwater divers
This is a list of underwater divers whose exploits have made them notable.
Underwater divers are people who take part in underwater diving activities – Underwater diving is practiced as part of an occupation, or for recreation, where t ...
and submariners also rely on artificially delivered . Submarines, submersibles and atmospheric diving suits
An atmospheric diving suit (ADS) is a small one-person articulated submersible which resembles a suit of armour, with elaborate pressure joints to allow articulation while maintaining an internal pressure of one atmosphere. An ADS can enable di ...
usually operate at normal atmospheric pressure. Breathing air is scrubbed of carbon dioxide by chemical extraction and oxygen is replaced to maintain a constant partial pressure. Ambient pressure
Ambient or Ambiance or Ambience may refer to:
Music and sound
* Ambience (sound recording), also known as atmospheres or backgrounds
* Ambient music, a genre of music that puts an emphasis on tone and atmosphere
* ''Ambient'' (album), by Moby
* ...
divers breathe air or gas mixtures with an oxygen fraction suited to the operating depth. Pure or nearly pure use in diving at pressures higher than atmospheric is usually limited to rebreathers, or decompression at relatively shallow depths (~6 meters depth, or less), or medical treatment in recompression chambers at pressures up to 2.8 bar, where acute oxygen toxicity can be managed without the risk of drowning. Deeper diving requires significant dilution of with other gases, such as nitrogen or helium, to prevent oxygen toxicity
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen () at increased partial pressures. Severe cases can result in cell damage and death, with effects most often seen in the central nervous system, lu ...
.
People who climb mountains or fly in non-pressurized fixed-wing aircraft sometimes have supplemental supplies.The reason is that increasing the proportion of oxygen in the breathing gas at low pressure acts to augment the inspired partial pressure nearer to that found at sea-level. Pressurized commercial airplanes have an emergency supply of automatically supplied to the passengers in case of cabin depressurization. Sudden cabin pressure loss activates chemical oxygen generator
A chemical oxygen generator is a device that releases oxygen via a chemical reaction. The oxygen source is usually an inorganic superoxide, chlorate, or perchlorate; ozonides are a promising group of oxygen sources. The generators are usually ig ...
s above each seat, causing oxygen masks to drop. Pulling on the masks "to start the flow of oxygen" as cabin safety instructions dictate, forces iron filings into the sodium chlorate
Sodium chlorate is an inorganic compound with the chemical formula Na ClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Sever ...
inside the canister. A steady stream of oxygen gas is then produced by the exothermic reaction.
Oxygen, as a mild euphoric
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and da ...
, has a history of recreational use in oxygen bar
An oxygen bar is an establishment, or part of one, that sells oxygen for recreational use. Individual scents may be added to enhance the experience. The flavors in an oxygen bar come from bubbling oxygen through bottles containing aromatic solutio ...
s and in sport
Sport pertains to any form of competitive physical activity or game that aims to use, maintain, or improve physical ability and skills while providing enjoyment to participants and, in some cases, entertainment to spectators. Sports can, ...
s. Oxygen bars are establishments found in the United States since the late 1990s that offer higher than normal exposure for a minimal fee. Professional athletes, especially in American football
American football (referred to simply as football in the United States and Canada), also known as gridiron, is a team sport played by two teams of eleven players on a rectangular field with goalposts at each end. The offense, the team wi ...
, sometimes go off-field between plays to don oxygen masks to boost performance. The pharmacological effect is doubted; a placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures.
In general, placebos can af ...
effect is a more likely explanation. Available studies support a performance boost from oxygen enriched mixtures only if it is inhaled ''during'' aerobic exercise
Aerobic exercise (also known as endurance activities, cardio or cardio-respiratory exercise) is physical exercise of low to high intensity that depends primarily on the aerobic energy-generating process. "Aerobic" is defined as "relating to, inv ...
.
Other recreational uses that do not involve breathing include pyrotechnic
Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. ...
applications, such as George Goble's five-second ignition of barbecue grills.
Industrial

Smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a ...
of iron ore into steel consumes 55% of commercially produced oxygen. In this process, is injected through a high-pressure lance into molten iron, which removes sulfur impurities and excess carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
as the respective oxides, and . The reactions are exothermic, so the temperature increases to 1,700 ° C.
Another 25% of commercially produced oxygen is used by the chemical industry. Ethylene is reacted with to create ethylene oxide
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sw ...
, which, in turn, is converted into ethylene glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
; the primary feeder material used to manufacture a host of products, including antifreeze
An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes also increase the boiling point of the liquid, all ...
and polyester polymers (the precursors of many plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptab ...
s and fabric
Textile is an umbrella term that includes various fiber-based materials, including fibers, yarns, filaments, threads, different fabric types, etc. At first, the word "textiles" only referred to woven fabrics. However, weaving is not th ...
s). Large quantities of oxygen or air is used in oxy-cracking process and for the production of acrylic acid, diformyl-furane, and benzylic acid. On the other hand, the electrochemical synthesis of hydrogen peroxide from oxygen is a promising technology to replace the currently used hydroquinone-process. Last but not least, catalytic oxidation is used in afterburners to get rid of hazardous gases.
Most of the remaining 20% of commercially produced oxygen is used in medical applications, metal cutting and welding, as an oxidizer in rocket fuel
Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical ...
, and in water treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, inc ...
. Oxygen is used in oxyacetylene welding
Principle of burn cutting
Oxy-fuel welding (commonly called oxyacetylene welding, oxy welding, or gas welding in the United States) and oxy-fuel cutting are processes that use fuel gases (or liquid fuels such as gasoline or petrol, diesel, ...
, burning acetylene with to produce a very hot flame. In this process, metal up to thick is first heated with a small oxy-acetylene flame and then quickly cut by a large stream of . Cook & Lauer 1968, p. 508
Compounds
 The
The oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
s. Compounds containing oxygen in other oxidation states are very uncommon: −1/2 (superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
s), −1/3 (ozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides.
Ionic ozonides
Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule.
Ino ...
s), 0 (elemental
An elemental is a mythic being that is described in occult and alchemical works from around the time of the European Renaissance, and particularly elaborated in the 16th century works of Paracelsus. According to Paracelsus and his subsequent fo ...
, hypofluorous acid
Hypofluorous acid, chemical formula H O F, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in hypofluorite ...
), +1/2 ( dioxygenyl), +1 ( dioxygen difluoride), and +2 (oxygen difluoride
Oxygen difluoride is a chemical compound with the formula . As predicted by VSEPR theory, the molecule adopts a "bent" molecular geometry. It is strong oxidizer and has attracted attention in rocketry for this reason. With a boiling point of -144.7 ...
).
Oxides and other inorganic compounds
Water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
() is an oxide of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and the most familiar oxygen compound. Hydrogen atoms are covalently bonded
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
to oxygen in a water molecule but also have an additional attraction (about 23.3 kJ/mol per hydrogen atom) to an adjacent oxygen atom in a separate molecule. These hydrogen bonds between water molecules hold them approximately 15% closer than what would be expected in a simple liquid with just van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s.Also, since oxygen has a higher electronegativity than hydrogen, the charge difference makes it a polar molecule
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
. The interactions between the different dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system ...
s of each molecule cause a net attraction force.
 Due to its
Due to its electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, oxygen forms chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s with almost all other elements to give corresponding oxides. The surface of most metals, such as aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
and titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
, are oxidized in the presence of air and become coated with a thin film of oxide that passivates the metal and slows further corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
. Many oxides of the transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s are non-stoichiometric compound
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); m ...
s, with slightly less metal than the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
would show. For example, the mineral FeO (wüstite
Wüstite ( Fe O) is a mineral form of iron(II) oxide found with meteorites and native iron. It has a grey colour with a greenish tint in reflected light. Wüstite crystallizes in the isometric-hexoctahedral crystal system in opaque to translu ...
) is written as , where ''x'' is usually around 0.05.
Oxygen is present in the atmosphere in trace quantities in the form of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
(). The Earth's crustal rock
Rock most often refers to:
* Rock (geology), a naturally occurring solid aggregate of minerals or mineraloids
* Rock music, a genre of popular music
Rock or Rocks may also refer to:
Places United Kingdom
* Rock, Caerphilly, a location in Wales ...
is composed in large part of oxides of silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
(silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
, as found in granite
Granite () is a coarse-grained ( phaneritic) intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly cools and solidifies under ...
and quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
), aluminium ( aluminium oxide , in bauxite
Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO ...
and corundum), iron ( iron(III) oxide , in hematite and rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO( ...
), and calcium carbonate (in limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms whe ...
). The rest of the Earth's crust is also made of oxygen compounds, in particular various complex silicates (in silicate minerals
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, silica (silicon dioxide, ) is usually con ...
). The Earth's mantle, of much larger mass than the crust, is largely composed of silicates of magnesium and iron.
Water-soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
silicates in the form of , , and are used as detergents and adhesives. Cook & Lauer 1968, p. 507
Oxygen also acts as a ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
for transition metals, forming transition metal dioxygen complexes, which feature metal–. This class of compounds includes the heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
proteins hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
and myoglobin. An exotic and unusual reaction occurs with , which oxidizes oxygen to give O2+PtF6−, dioxygenyl hexafluoroplatinate
Dioxygenyl hexafluoroplatinate is a compound with formula O2PtF6. It is a hexafluoroplatinate of the unusual dioxygenyl cation, O2+, and is the first known compound containing this cation. It can be produced by the reaction of dioxygen with platin ...
. Cook & Lauer 1968, p.505
Organic compounds
 Among the most important classes of organic compounds that contain oxygen are (where "R" is an organic group): alcohols (R-OH);
Among the most important classes of organic compounds that contain oxygen are (where "R" is an organic group): alcohols (R-OH); ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
s (R-O-R); ketones (R-CO-R); aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s (R-CO-H); carboxylic acids (R-COOH); ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s (R-COO-R); acid anhydrides
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
(R-CO-O-CO-R); and amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
s (). There are many important organic solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s that contain oxygen, including: acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
, methanol, ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
, isopropanol, furan, THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
, diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
, dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
, ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
, DMF, DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds ...
, acetic acid, and formic acid. Acetone () and phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
() are used as feeder materials in the synthesis of many different substances. Other important organic compounds that contain oxygen are: glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
, formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
, glutaraldehyde
Glutaraldehyde is an organic compound with the formula . The molecule consists of a five carbon chain doubly terminated with formyl (CHO) groups. It is usually used as a solution in water, and such solutions exists as a collection of hydrates, c ...
, citric acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in ...
, acetic anhydride, and acetamide
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound ''N'',''N''-dimet ...
. Epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
s are ethers in which the oxygen atom is part of a ring of three atoms. The element is similarly found in almost all biomolecules that are important to (or generated by) life.
Oxygen reacts spontaneously with many organic compounds at or below room temperature in a process called autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
. Cook & Lauer 1968, p. 506 Most of the organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s that contain oxygen are not made by direct action of . Organic compounds important in industry and commerce that are made by direct oxidation of a precursor include ethylene oxide
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sw ...
and peracetic acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the chemical formula, formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corros ...
.
Safety and precautions
TheNFPA 704
"NFPA 704: Standard System for the Identification of the Hazards of Materials for Emergency Response" is a standard maintained by the U.S.-based National Fire Protection Association. First "tentatively adopted as a guide" in 1960, and revised se ...
standard rates compressed oxygen gas as nonhazardous to health, nonflammable and nonreactive, but an oxidizer. Refrigerated liquid oxygen (LOX) is given a health hazard rating of 3 (for increased risk of hyperoxia
Hyperoxia occurs when cells, tissues and organs are exposed to an excess supply of oxygen (O2) or higher than normal partial pressure of oxygen.
In medicine, it refers to excessive oxygen in the lungs or other body tissues, which can be caused ...
from condensed vapors, and for hazards common to cryogenic liquids such as frostbite), and all other ratings are the same as the compressed gas form.
Toxicity
 Oxygen gas () can be
Oxygen gas () can be toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
at elevated partial pressures, leading to convulsions and other health problems.Since 's partial pressure is the fraction of times the total pressure, elevated partial pressures can occur either from high fraction in breathing gas or from high breathing gas pressure, or a combination of both. Cook & Lauer 1968, p. 511 Oxygen toxicity usually begins to occur at partial pressures more than 50 kilopascals
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined ...
(kPa), equal to about 50% oxygen composition at standard pressure or 2.5 times the normal sea-level partial pressure of about 21 kPa. This is not a problem except for patients on mechanical ventilator
A ventilator is a piece of medical technology that provides mechanical ventilation by moving breathable air into and out of the lungs, to deliver breaths to a patient who is physically unable to breathe, or breathing insufficiently. Ventilators ...
s, since gas supplied through oxygen masks in medical applications is typically composed of only 30–50% by volume (about 30 kPa at standard pressure).
At one time, premature babies
Preterm birth, also known as premature birth, is the birth of a baby at fewer than 37 weeks gestational age, as opposed to full-term delivery at approximately 40 weeks. Extreme preterm is less than 28 weeks, very early preterm birth is between ...
were placed in incubators containing -rich air, but this practice was discontinued after some babies were blinded by the oxygen content being too high.
Breathing pure in space applications, such as in some modern space suits, or in early spacecraft such as Apollo
Apollo, grc, Ἀπόλλωνος, Apóllōnos, label=genitive , ; , grc-dor, Ἀπέλλων, Apéllōn, ; grc, Ἀπείλων, Apeílōn, label= Arcadocypriot Greek, ; grc-aeo, Ἄπλουν, Áploun, la, Apollō, la, Apollinis, label ...
, causes no damage due to the low total pressures used. In the case of spacesuits, the partial pressure in the breathing gas is, in general, about 30 kPa (1.4 times normal), and the resulting partial pressure in the astronaut's arterial blood is only marginally more than normal sea-level partial pressure.
Oxygen toxicity to the lungs and central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all p ...
can also occur in deep scuba diving
Scuba diving is a mode of underwater diving whereby divers use breathing equipment that is completely independent of a surface air supply. The name "scuba", an acronym for " Self-Contained Underwater Breathing Apparatus", was coined by Chr ...
and surface supplied diving. Prolonged breathing of an air mixture with an partial pressure more than 60 kPa can eventually lead to permanent pulmonary fibrosis
Pulmonary fibrosis is a condition in which the lungs become scarred over time. Symptoms include shortness of breath, a dry cough, feeling tired, weight loss, and nail clubbing. Complications may include pulmonary hypertension, respiratory failu ...
. Exposure to an partial pressures greater than 160 kPa (about 1.6 atm) may lead to convulsions (normally fatal for divers). Acute oxygen toxicity (causing seizures, its most feared effect for divers) can occur by breathing an air mixture with 21% at or more of depth; the same thing can occur by breathing 100% at only .
Combustion and other hazards
 Highly concentrated sources of oxygen promote rapid combustion.
Highly concentrated sources of oxygen promote rapid combustion. Fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames a ...
and explosion hazards exist when concentrated oxidants and fuels are brought into close proximity; an ignition event, such as heat or a spark, is needed to trigger combustion. Oxygen is the oxidant, not the fuel.
Concentrated will allow combustion to proceed rapidly and energetically. Steel pipes and storage vessels used to store and transmit both gaseous and liquid oxygen
Liquid oxygen—abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries—is the liquid form of molecular oxygen. It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an app ...
will act as a fuel; and therefore the design and manufacture of systems requires special training to ensure that ignition sources are minimized. The fire that killed the Apollo 1
Apollo 1, initially designated AS-204, was intended to be the first crewed mission of the Apollo program, the American undertaking to land the first man on the Moon. It was planned to launch on February 21, 1967, as the first low Earth orbita ...
crew in a launch pad test spread so rapidly because the capsule was pressurized with pure but at slightly more than atmospheric pressure, instead of the normal pressure that would be used in a mission.
Liquid oxygen spills, if allowed to soak into organic matter, such as wood
Wood is a porous and fibrous structural tissue found in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulose fibers that are strong in tension and embedded in a matrix of lignin ...
, petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable so ...
s, and asphalt
Asphalt, also known as bitumen (, ), is a sticky, black, highly viscous liquid or semi-solid form of petroleum. It may be found in natural deposits or may be a refined product, and is classed as a pitch. Before the 20th century, the term ...
can cause these materials to Detonation, detonate unpredictably on subsequent mechanical impact.
See also
* Geological history of oxygen * Hypoxia (environmental) for depletion in aquatic ecology * Ocean deoxygenation * Hypoxia (medical), a lack of oxygen * Limiting oxygen concentration * :Oxygen compounds, Oxygen compounds * Oxygen plant * Oxygen sensorNotes
References
General references
* * *External links
Oxygen
at ''The Periodic Table of Videos'' (University of Nottingham)
Oxidizing Agents > Oxygen
Roald Hoffmann article on "The Story of O"
*
Scripps Institute: Atmospheric Oxygen has been dropping for 20 years
{{featured article Oxygen, Chemical elements Diatomic nonmetals Reactive nonmetals Chalcogens Chemical substances for emergency medicine Breathing gases E-number additives Oxidizing agents