Ethylene oxide is an
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. It is a cyclic
ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again ...
and the simplest
epoxide: a three-membered
ring consisting of one
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
atom and two
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
atoms. Ethylene oxide is a colorless and
flammable
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable mat ...
gas with a faintly sweet odor. Because it is a
strained ring
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are ...
, ethylene oxide easily participates in a number of
addition reactions that result in ring-opening. Ethylene oxide is
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Is ...
ic with
acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
and with
vinyl alcohol. Ethylene oxide is industrially produced by
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
of
ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
in the presence of
silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
.
The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as
ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an o ...
, ethanolamines, simple and complex glycols,
polyglycol ethers, and other compounds. Although it is a vital raw material with diverse applications, including the manufacture of products like
polysorbate 20 and
polyethylene glycol (PEG) that are often more effective and less toxic than alternative materials, ethylene oxide itself is a very hazardous substance. At room temperature it is a flammable,
carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
,
mutagenic, irritating, and
anaesthetic gas.
[
Ethylene oxide is a surface ]disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than s ...
that is widely used in hospitals and the medical equipment industry to replace steam in the sterilization of heat-sensitive tools and equipment, such as disposable plastic syringes. It is so flammable and extremely explosive that it is used as a main component of thermobaric weapons;[ therefore, it is commonly handled and shipped as a refrigerated liquid to control its hazardous nature.][Rebsdat, Siegfried and Mayer, Dieter (2005) "Ethylene Oxide" in ''Ullmann's Encyclopedia of Industrial Chemistry''. Wiley-VCH, Weinheim. .]
History
Ethylene oxide was first reported in 1859 by the French
French (french: français(e), link=no) may refer to:
* Something of, from, or related to France
** French language, which originated in France, and its various dialects and accents
** French people, a nation and ethnic group identified with Franc ...
chemist Charles-Adolphe Wurtz
Charles Adolphe Wurtz (; 26 November 181710 May 1884) was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinio ...
, who prepared it by treating 2-chloroethanol
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the ''simplest'' beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like o ...
with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
:
:Cl-CH2CH2-OH + KOH -> (CH2CH2)O + KCl + H2O
Wurtz measured the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding env ...
of ethylene oxide as , slightly higher than the present value, and discovered the ability of ethylene oxide to react with acids and salts of metals.electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
.ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again ...
s — particularly by its propensity to engage in the addition reactions typical of unsaturated compounds — had long been a matter of debate. The heterocyclic triangular structure of ethylene oxide was proposed by 1868 or earlier.
Wurtz's 1859 synthesis long remained the only method of preparing ethylene oxide, despite numerous attempts, including by Wurtz himself, to produce ethylene oxide directly from ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
.silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. Since 1940, almost all industrial production of ethylene oxide has relied on this process. Sterilization by ethylene oxide for the preservation of spice
A spice is a seed, fruit, root, bark, or other plant substance primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of plants used for flavoring or as a garnish. Spices a ...
s was patented in 1938 by the American
American(s) may refer to:
* American, something of, from, or related to the United States of America, commonly known as the "United States" or "America"
** Americans, citizens and nationals of the United States of America
** American ancestry, pe ...
chemist Lloyd Hall. Ethylene oxide achieved industrial importance during World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was List of wars and anthropogenic disasters by death toll, one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, ...
as a precursor to both the coolant ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an o ...
and the chemical weapon mustard gas
Mustard gas or sulfur mustard is a chemical compound belonging to a family of cytotoxic and blister agents known as mustard agents. The name ''mustard gas'' is technically incorrect: the substance, when dispersed, is often not actually a gas, ...
.
Molecular structure and properties

 The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol.
The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol.ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again ...
s, the C–O–C angle is 120°. The moment of inertia
The moment of inertia, otherwise known as the mass moment of inertia, angular mass, second moment of mass, or most accurately, rotational inertia, of a rigid body is a quantity that determines the torque needed for a desired angular accele ...
about each of the principal axes are ''IA'' = , ''IB'' = and ''IC'' = .
The relative instability of the carbon-oxygen bonds in the molecule is revealed by the comparison in the table of the energy required to break two C–O bonds in the ethylene oxide or one C–O bond in ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
and dimethyl ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3,
(sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor ...
:
This instability correlates with its high reactivity, explaining the ease of its ring-opening reactions (see Chemical properties).
Physical properties
Ethylene oxide is a colorless gas at and is a mobile liquid at – viscosity of liquid ethylene oxide at 0 °C is about 5.5 times lower than that of water. The gas has a characteristic sweet odor of ether, noticeable when its concentration in air exceeds 500ppm.ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
, diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
and many organic solvents.
Main thermodynamical constants are:surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) t ...
of liquid ethylene oxide, at the interface with its own vapor, is at and at .
* The boiling point increases with the vapor pressure as follows: (), (), and ().
* Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the int ...
decreases with temperature with the values of 0.577kPa·s at , 0.488 kPa·s at , 0.394kPa·s at , and 0.320kPa·s at .
Between , vapor pressure ''p'' (in mmHg) varies with temperature (''T'' in °C) as
:.
*N/A – data not available.
*N/A – data not available.
Chemical properties
Ethylene oxide readily reacts with diverse compounds with opening of the ring. Its typical reactions are with nucleophiles which proceed via the SN2 mechanism both in acidic (weak nucleophiles: water, alcohols) and alkaline media (strong nucleophiles: OH−, RO−, NH3, RNH2, RR'NH, etc.).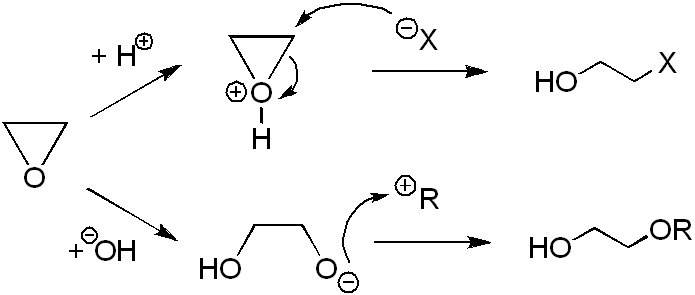 and more specific reactions are described below.
and more specific reactions are described below.
Addition of water and alcohols
Aqueous solutions of ethylene oxide are rather stable and can exist for a long time without any noticeable chemical reaction, but adding a small amount of acid, such as strongly diluted sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
, immediately leads to the formation of ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an o ...
, even at room temperature:
: (CH2CH2)O + H2O → HO–CH2CH2–OH
The reaction also occurs in the gas phase, in the presence of a phosphoric acid salt as a catalyst.sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
metal, sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and al ...
or boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bond ...
and are used for the synthesis of surfactants.
Addition of carboxylic acids and their derivatives
Reactions of ethylene oxide with carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
s in the presence of a catalyst results in glycol mono- and diesters:
: (CH2CH2)O + CH3CO2H → HOCH2CH2–O2CCH3
: (CH2CH2)O + (CH3CO)2O → CH3CO2CH2CH2O2CCH3
The addition of acid amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
s proceeds similarly:
: (CH2CH2)O + CH3CONH2 → HOCH2CH2NHC(O)CH3
Addition of ethylene oxide to higher carboxylic acids is carried out at elevated temperatures (typically ) and pressure () in an inert atmosphere, in presence of an alkaline catalyst (concentration 0.01–2%), such as hydroxide or carbonate of sodium or potassium. The carboxylate ion acts as nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
in the reaction:
: (CH2CH2)O + RCO2− → RCO2CH2CH2O−
:RCO2CH2CH2O− + RCO2H → RCO2CH2CH2OH + RCO2−
Adding ammonia and amines
Ethylene oxide reacts with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
forming a mixture of mono-, di- and tri- ethanolamines. The reaction is stimulated by adding a small amount of water.
: (CH2CH2)O + NH3 → HO–CH2CH2–NH2
:2 (CH2CH2)O + NH3 → (HO–CH2CH2)2NH
:3 (CH2CH2)O + NH3 → (HO–CH2CH2)3N
Similarly proceed the reactions with primary and secondary amines:
: (CH2CH2)O + RNH2 → HO–CH2CH2–NHR
Dialkylamino ethanols can further react with ethylene oxide, forming amino polyethylene glycols:
Halide addition
Ethylene oxide readily reacts with aqueous solutions of hydrochloric, hydrobromic and hydroiodic acids to form halohydrin
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol ...
s. The reaction occurs easier with the last two acids:
: (CH2CH2)O + HCl → HO–CH2CH2–Cl
The reaction with these acids competes with the acid-catalyzed hydration of ethylene oxide; therefore, there is always a by-product of ethylene glycol with an admixture of diethylene glycol. For a cleaner product, the reaction is conducted in the gas phase or in an organic solvent.
Ethylene fluorohydrin is obtained differently, by boiling hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock ...
with a 5–6% solution of ethylene oxide in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
. The ether normally has a water content of 1.5–2%; in absence of water, ethylene oxide polymerizes.
Halohydrins can also be obtained by passing ethylene oxide through aqueous solutions of metal halides:
Metalorganic addition
Interaction of ethylene oxide with organomagnesium compounds, which are Grignard reagents, can be regarded as nucleophilic substitution influenced by carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3 ...
organometallic compounds. The final product of the reaction is a primary alcohol:
: (CH2CH2)O + RMgBr -> R-CH2CH2-OMgBr -> ce\overset
Similar mechanism is valid for other organometallic compounds, such as alkyl lithium:
: (CH2CH2)O + \overset -> R-CH2CH2-OLi -> ceR-CH2CH2-OH
Other addition reactions
Addition of hydrogen cyanide
Ethylene oxide easily reacts with hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on a ...
forming ethylene cyanohydrin:
: (CH2CH2)O + HCN → HO–CH2CH2–CN
A slightly chilled (10–20 °C) aqueous solution of calcium cyanide can be used instead of HCN:
:2 (CH2CH2)O + Ca(CN)2 + 2 H2O → 2 HO–CH2CH2–CN + Ca(OH)2
Ethylene cyanohydrin easily loses water, producing acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecula ...
:
:HO–CH2CH2–CN → CH2=CH–CN + H2O
Addition of hydrogen sulfide and mercaptans
When reacting with the hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
, ethylene oxide forms 2-mercaptoethanol and thiodiglycol, and with alkylmercaptans it produces 2-alkyl mercaptoetanol:
: (CH2CH2)O + H2S → HO–CH2CH2–HS
:2 (CH2CH2)O + H2S → (HO–CH2CH2)2S
: (CH2CH2)O + RHS → HO–CH2CH2–SR
The excess of ethylene oxide with an aqueous solution of hydrogen sulfide leads to the tris-(hydroxyethyl) sulfonyl hydroxide:
:3 (CH2CH2)O + H2S → HO–CH2CH2)3S+H−
Addition of nitrous and nitric acids
Reaction of ethylene oxide with aqueous solutions of barium nitrite, calcium nitrite, magnesium nitrite, zinc nitrite or sodium nitrite
Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite ...
leads to the formation of 2-nitroethanol:
:2 (CH2CH2)O + Ca(NO2)2 + 2 H2O → 2 HO–CH2CH2–NO2 + Ca(OH)2
With nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, ethylene oxide forms mono- and dinitroglycols:
: (CH2CH2)O + \overset -> HO-CH2CH2-ONO2 -> ce ceO2NO-CH2CH2-ONO_2
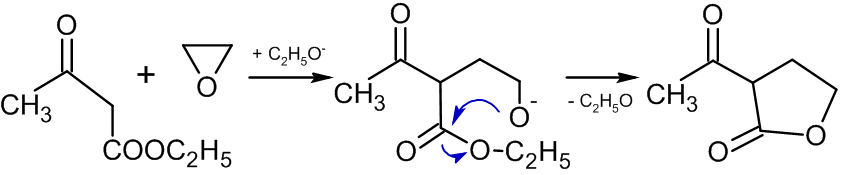
Reaction with compounds containing active methylene groups
In the presence of alkoxides, reactions of ethylene oxide with compounds containing active methylene group leads to the formation of butyrolactones:
: 
Alkylation of aromatic compounds
Ethylene oxide enters into the Friedel–Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation react ...
with benzene to form phenethyl alcohol
Phenethyl alcohol, or 2-phenylethanol, is the organic compound that consists of a phenethyl group (C6H5CH2CH2) attached to OH. It is a colourless liquid that is slightly soluble in water (2 ml/100 ml H2O), but miscible with most organic solvents. ...
:
:  Styrene can be obtained in one stage if this reaction is conducted at elevated temperatures () and pressures (), in presence of an aluminosilicate catalyst.
Styrene can be obtained in one stage if this reaction is conducted at elevated temperatures () and pressures (), in presence of an aluminosilicate catalyst.
Synthesis of crown ethers
A series of polynomial heterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, an ...
s, known as crown ethers, can be synthesized with ethylene oxide. One method is the cationic cyclopolymerization of ethylene oxide, limiting the size of the formed cycle:sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
in the presence of caesium salts leads to the formation of an 11-membered heterocyclic compound which has the complexing properties of crown ethers: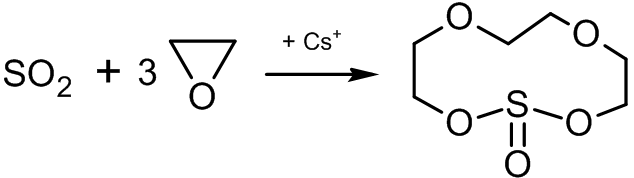
Isomerization
When heated to about , or to in the presence of a catalyst ( Al2O3, H3PO4, etc.), ethylene oxide isomerizes into acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
:binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
of the C-C bond in acetaldehyde.
Reduction reaction
Ethylene oxide can be hydrogenated into ethanol in the presence of a catalyst, such as nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
, platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
, palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
,lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
and some other hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s.acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
, of lithium aluminium hydride with titanium trichloride (the reducing agent is actually titanium dichloride, formed by the reaction between LiAlH4 and TiCl3) and of iron(III) chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The col ...
with butyllithium in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
.
Oxidation
Ethylene oxide can further be oxidized, depending on the conditions, to glycolic acid or carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
:
: (CH2CH2)O + O2 -> ce\overset
Deep gas-phase reactor oxidation of ethylene oxide at and a pressure of yields a complex mixture of products containing O2, H2, CO, CO2, CH4, C2H2, C2H4, C2H6, C3H6, C3H8 and CH3CHO.
Dimerization
In the presence of acid catalysts, ethylene oxide dimerizes to afford dioxane:
: 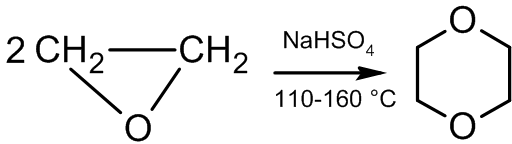 The reaction mechanism is as follows:
The reaction mechanism is as follows: The dimerization reaction is unselective. By-products include
The dimerization reaction is unselective. By-products include acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
(due to isomerization). The selectivity and speed of dimerization can be increased by adding a catalyst, such as platinum, platinum-palladium, or iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
with sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solve ...
. 2-methyl-1,3-dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2- ...
is formed as a side product in the last case.
Polymerization
Liquid ethylene oxide can form polyethylene glycols. The polymerization can proceed via radical and ionic mechanisms, but only the latter has a wide practical application.protic
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labil ...
acids ( HClO4, HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a s ...
), Lewis acids ( SnCl4, BF3, etc.), organometallic compounds, or more complex reagents:alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s, hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
s, carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
s or other compounds of alkali or alkaline earth metals.
Thermal decomposition
Ethylene oxide is relatively stable to heating – in the absence of a catalyst, it does not dissociate up to , and only above there is a major exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
decomposition, which proceeds through the radical mechanism.formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
. High-temperature pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements '' ...
() at elevated pressure in an inert atmosphere leads to a more complex composition of the gas mixture, which also contains acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
and propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as ...
.
Other reactions
Thiocyanate ions or thiourea transform ethylene oxide into thiirane (ethylene sulfide):
: (CH2CH2)O + (NH2)2C=S → (CH2CH2)S + (NH2)2C=O
:  Reaction of
Reaction of phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and mois ...
with ethylene oxide produces ethylene dichloride:pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
and of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
and carbon tetrachloride.[
]
Phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic ...
reacts with ethylene oxide forming chloroethyl esters of phosphorous acid:acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s in the presence of sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I ...
is a complex iodoethyl ester:carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, in a non-polar solvent in the presence of ''bis''-(triphenylphosphine)-nickel(0) results in ethylene carbonate:
:  In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with
In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
at 80–150 °C in the presence of a catalyst leads to the formation of 1,3-dioxolane: Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon ...
of ethylene oxide gives hydroxypropanal which can be hydrogenated to propane-1,3-diol:
: (CH2CH2)O + CO + H2 -> CHO-CH2CH2-OH -> ceHO-CH2CH2CH2-OH
Laboratory synthesis
Dehydrochlorination of ethylene and its derivatives
Dehydrochlorination of 2-chloroethanol
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the ''simplest'' beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like o ...
, developed by Wurtz in 1859, remains a common laboratory route to ethylene oxide:
:Cl-CH2CH2-OH + NaOH -> (CH2CH2)O + NaCl + H2O
The reaction is carried out at elevated temperature, and beside sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and al ...
or potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
, calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has ma ...
, barium hydroxide, magnesium hydroxide or carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
s of alkali or alkaline earth metals can be used.calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic ...
with ethyl hypochlorite; substituting calcium by other alkaline earth metals reduces the reaction yield:
Direct oxidation of ethylene by peroxy acids
Ethylene can be directly oxidized into ethylene oxide using peroxy acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the perox ...
s, for example, peroxybenzoic or ''meta''-chloro-peroxybenzoic acid:
:  Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.
Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.
Other preparative methods
Other synthesis methods include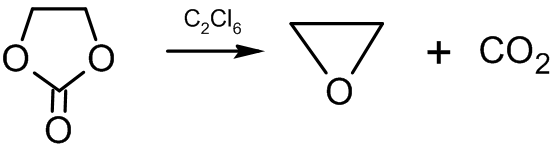
Industrial synthesis
History
Commercial production of ethylene oxide dates back to 1914 when BASF built the first factory which used the chlorohydrin process (reaction of ethylene chlorohydrin with calcium hydroxide). The chlorohydrin process was unattractive for several reasons, including low efficiency and loss of valuable chlorine into calcium chloride. More efficient direct oxidation of ethylene by air was invented by Lefort in 1931 and in 1937 Union Carbide
Union Carbide Corporation is an American chemical corporation wholly owned subsidiary (since February 6, 2001) by Dow Chemical Company. Union Carbide produces chemicals and polymers that undergo one or more further conversions by customers befo ...
opened the first plant using this process. It was further improved in 1958 by Shell Oil Co. by replacing air with oxygen and using elevated temperature of and pressure ().
Chlorohydrin process of production of ethylene oxide
Although the chlorohydrin process is almost entirely superseded in the industry by the direct oxidation of ethylene, the knowledge of this method is still important for educational reasons and because it is still used in the production of propylene oxide. The process consists of three major steps: synthesis of ethylene chlorohydrin, dehydrochlorination of ethylene chlorohydrin to ethylene oxide and purification of ethylene oxide. Those steps are carried continuously. In the first column, hypochlorination of ethylene is carried out as follows:rectification
Rectification has the following technical meanings:
Mathematics
* Rectification (geometry), truncating a polytope by marking the midpoints of all its edges, and cutting off its vertices at those points
* Rectifiable curve, in mathematics
* Recti ...
. The chlorohydrin process allows to reach 95% conversion of ethylene chlorohydrin. The yield of ethylene oxide is about 80% of the theoretical value; for of ethylene oxide, about of ethylene dichloride is produced.
Direct oxidation of ethylene
Usage in global industry
Direct oxidation of ethylene was patented by Lefort in 1931. This method was repeatedly modified for industrial use, and at least four major variations are known. They all use oxidation by oxygen or air and a silver-based catalyst, but differ in the technological details and hardware implementations.Union Carbide
Union Carbide Corporation is an American chemical corporation wholly owned subsidiary (since February 6, 2001) by Dow Chemical Company. Union Carbide produces chemicals and polymers that undergo one or more further conversions by customers befo ...
(currently a division of Dow Chemical Company
The Dow Chemical Company, officially Dow Inc., is an American multinational chemical corporation headquartered in Midland, Michigan, United States. The company is among the three largest chemical producers in the world.
Dow manufactures plastics ...
) was the first company to develop the direct oxidation process.
Chemistry and kinetics of the direct oxidation process
Formally, the direct oxidation process is expressed by the following equation:
: 2CH_2=CH2 + O2 -> ce2(CH2CH2)O, ΔH = −105 kJ/mol
However, significant yield of carbon dioxide and water is observed in practice, which can be explained by the complete oxidation of ethylene or ethylene oxide:
: CH2=CH2 + 3 O2 → 2 CO2 + 2 H2O, ΔH = −1327kJ/mol
: (CH2CH2)O + 2.5 O2 → 2 CO2 + 2 H2O, ΔH = −1223kJ/mol
According to a kinetic analysis by Kilty and Sachtler, the following reactions describe the pathway leading to EO. In the first step, a superoxide (O2−) species is formed:pumice
Pumice (), called pumicite in its powdered or dust form, is a volcanic rock that consists of highly vesicular rough-textured volcanic glass, which may or may not contain crystals. It is typically light-colored. Scoria is another vesicular v ...
, silica gel, various silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is a ...
s and aluminosilicates, alumina and silicon carbide, and activated by certain additives (antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient ti ...
, bismuth, barium peroxide, etc.).
Process overview
The production of ethylene oxide on a commercial scale is attained with the unification of the following unit processes:
* Main reactor
* Ethylene oxide scrubber
Scrubber systems (e.g. chemical scrubbers, gas scrubbers) are a diverse group of air pollution control devices that can be used to remove some particulates and/or gases from industrial exhaust streams. An early application of a carbon dioxide sc ...
* Ethylene oxide de-sorber
* Stripping and distillation column
A fractionating column or fractional column is an essential item used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on the differences in volatilities. Fractionating columns are used in ...
* CO2 scrubber and CO2 de-scrubber
Main Reactor: The main reactor consists of thousands of catalyst tubes in bundles. These tubes are generally long with an inner diameter of . The catalyst packed in these tubes is in the form of spheres or rings of diameter . The operating conditions of with a pressure of prevail in the reactor. To maintain this temperature, the cooling system of the reactor plays a vital role. With the aging of the catalyst, its selectivity decreases and it produces more exothermic side products of CO2.
Ethylene oxide scrubber: After the gaseous stream from the main reactor, containing ethylene oxide (1–2%) and CO2 (5%), is cooled, it is then passed to the ethylene oxide scrubber. Here, water is used as the scrubbing media which scrubs away majority of ethylene oxide along with some amounts of CO2, N2, CH2=CH2, CH4 and aldehydes (introduced by the recycle stream). Also, a small proportion of the gas leaving the ethylene oxide scrubber (0.1–0.2%) is removed continuously (combusted) to prevent the buildup of inert compounds (N2, Ar, and C2H6), which are introduced as impurities with the reactants.
Ethylene oxide de-sorber: The aqueous stream resulting from the above scrubbing process is then sent to the ethylene oxide de-sorber. Here, ethylene oxide is obtained as the overhead product, whereas the bottom product obtained is known as the ''glycol bleed''. When ethylene oxide is scrubbed from the recycle gas with an aqueous solution, ethylene glycols (viz. mono-ethylene glycol, di-ethylene glycol and other poly-ethylene glycols) get unavoidably produced. Thus, in-order to prevent them from building up in the system, they are continuously bled off.
Stripping and distillation column: Here, the ethylene oxide stream is stripped off its low boiling components and then distilled in-order to separate it into water and ethylene oxide.
CO2 scrubber: The recycle stream obtained from the ethylene oxide scrubber is compressed and a side-stream is fed to the CO2 scrubber. Here, CO2 gets dissolved into the hot aqueous solution of potassium carbonate (i.e., the scrubbing media). The dissolution of CO2 is not only a physical phenomenon, but a chemical phenomenon as well, for, the CO2 reacts with potassium carbonate to produce potassium hydrogen carbonate.
: K2CO3 + CO2 + H2O → 2 KHCO3
CO2 de-scrubber: The above potassium carbonate solution (enriched with CO2) is then sent to the CO2 de-scrubber where CO2 is de-scrubbed by stepwise (usually two steps) flashing. The first step is done to remove the hydrocarbon gases, and the second step is employed to strip off CO2.
World production of ethylene oxide
The world production of ethylene oxide was in 2009,[ in 2008 and in 2007.]Dow Chemical Company
The Dow Chemical Company, officially Dow Inc., is an American multinational chemical corporation headquartered in Midland, Michigan, United States. The company is among the three largest chemical producers in the world.
Dow manufactures plastics ...
( in 2006Royal Dutch Shell
Shell plc is a British multinational oil and gas company headquartered in London, England. Shell is a public limited company with a primary listing on the London Stock Exchange (LSE) and secondary listings on Euronext Amsterdam and the New ...
( in 2008–2009), BASF ( in 2008–2009), China Petrochemical Corporation (~ in 2006
Applications
 Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of
Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an o ...
s, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include natura ...
and polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and food ...
(PET – raw material for plastic bottles), liquid coolants and solvents.
Polyethyleneglycols are used in perfumes, cosmetics, pharmaceuticals, lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, t ...
s, paint thinner
A paint thinner is a solvent used to thin oil-based paints. Solvents labeled "paint thinner" are usually mineral spirits having a flash point at about 40 °C (104 °F), the same as some popular brands of charcoal starter.
Common sol ...
s and plasticizers. Ethylene glycol ethers are part of brake fluids, detergents, solvents, lacquers and paints. Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols, acids or amines. They are used in the manufacture of detergents, surfactants, emulsifier
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althoug ...
s and dispersant
A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid (such as a colloid or emulsion) to improve the separation of the particles and to prevent their sett ...
s.
Whereas synthesis of ethylene glycols is the major application of ethylene oxide, its percentage varies greatly depending on the region: from 44% in the Western Europe
Western Europe is the western region of Europe. The region's countries and territories vary depending on context.
The concept of "the West" appeared in Europe in juxtaposition to "the East" and originally applied to the ancient Mediterranean ...
, 63% in Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the n ...
and 73% in North America
North America is a continent in the Northern Hemisphere and almost entirely within the Western Hemisphere. It is bordered to the north by the Arctic Ocean, to the east by the Atlantic Ocean, to the southeast by South America and th ...
to 90% in the rest of Asia
Asia (, ) is one of the world's most notable geographical regions, which is either considered a continent in its own right or a subcontinent of Eurasia, which shares the continental landmass of Afro-Eurasia with Africa. Asia covers an are ...
and 99% in Africa
Africa is the world's second-largest and second-most populous continent, after Asia in both cases. At about 30.3 million km2 (11.7 million square miles) including adjacent islands, it covers 6% of Earth's total surface area ...
.
Production of ethylene glycol
Ethylene glycol is industrially produced by non-catalytic hydration of ethylene oxide at a temperature of and a pressure of :phosphonium
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Types of phosphonium ...
halide as a catalyst. The glycol yield is 99–99.5%, with other glycols practically absent. The main advantage of the process is production of pure ethylene glycol without the need for further purification. The first commercial plant which uses this method was opened in 2008 in South Korea. Dow METEOR (Most Effective Technology for Ethylene Oxide Reactions) is an integrated technology for producing ethylene oxide and its subsequent hydrolysis into ethylene glycol. The glycol yield is 90–93%. The main advantage of the process is relative simplicity, using fewer stages and less equipment.
Conversion to ethylene glycol is also the means by which waste ethylene oxide is scrubbed before venting to the environment. Typically the EtO is passed over a matrix containing either sulfuric acid or potassium permanganate.
Production of glycol ethers
The major industrial esters of mono-, di- and triethylene glycols are methyl, ethyl and normal butyl ethers, as well as their acetates and phthalates. The synthesis involves reaction of the appropriate alcohol with ethylene oxide:
: (CH2CH2)O + ROH -> HOCH2CH2OR
: (CH2CH2)O + HOCH2CH2OR -> HOCH2CH2OCH2CH2OR
: (CH2CH2)O + HOCH2CH2OCH2CH2OR -> HOCH2CH2OCH2CH2OCH2CH2OR
The reaction of monoesters with an acid or its anhydride leads to the formation of the esters:
:CH3CO2H + HOCH2CH2OR -> ROCH2CH2OCOCH3 + H2O
Production of ethanolamines
In the industry, ethanolamines (mono-, di- and triethanolamines) are produced by reacting ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
and ethylene oxide in anhydrous medium at a temperature of and pressure of MPa:
:(CH2CH2)O + NH3 -> HOCH2CH2NH2
:2 (CH2CH2)O + NH3 -> (HOCH2CH2)2NH
:3 (CH2CH2)O + NH3 -> (HOCH2CH2)3N
All three ethanolamines are produced in the process, while ammonia and part of methylamine are recycled. The final products are separated by vacuum distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
. Hydroxyalkylamines are produced in a similar process:
:(CH2CH2)O + RNH2 -> HOCH2CH2NHR
:2 (CH2CH2)O + RNH2 -> (HOCH2CH2)2NR
Monosubstituted products are formed by reacting a large excess of amine with ethylene oxide in presence of water and at a temperature below . Disubstituted products are obtained with a small excess of ethylene oxide, at a temperature of and a pressure of .
Production of ethoxylates
Industrial production of ethoxylates is realized by a direct reaction of higher alcohols, acids or amines with ethylene oxide in the presence of an alkaline catalyst at a temperature of . Modern plants producing ethoxylates are usually based on the BUSS LOOP reactors technology,
Production of acrylonitrile
Currently, most acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecula ...
(90% in 2008) is produced by the SOHIO method, which is based on the catalytic oxidation of propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petro ...
in the presence of ammonia and bismuth phosphomolybdate. However, until 1960 a key production process was addition of hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on a ...
to ethylene oxide, followed by dehydration of the resulting cyanohydrin
In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is , where R is H, alkyl, or aryl. Cyanohyd ...
:sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and al ...
and diethylamine), and dehydration of cyanohydrin occurs in the gas phase upon the catalytic action of aluminium oxide.
Non-industrial uses
The direct use of ethylene oxide accounts for only 0.05% (2004 data) of its global production.
Healthcare sterilant
Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry because of its non-damaging effects for delicate instruments and devices that require sterilization, and for its wide range of material compatibility. It is used for instruments that cannot tolerate heat, moisture or abrasive chemicals, such as electronics, optical equipment, paper, rubber and plastics. It was developed in the 1940s as a sterilant by the US military, and its use as a medical sterilant dates to the late 1950s, when the McDonald process was patented for medical devices. The Anprolene system was patented in the 1960s by Andersen Products, and it remains the most commonly used system in several niche markets, notably the veterinary market and some international markets. It relies on the use of a flexible sterilization chamber and an EtO cartridge for small volume sterilization, and where environmental and/or portability considerations dictate the use of a low dose. It is therefore referred to as the "flexible chamber sterilization" method, or the "gas diffusion sterilization" method.
In the United States, the operation of EtO sterilization is overseen by the EPA
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
through the National Emissions Standards for Hazardous Air Pollutants (NESHAP).
Niche uses
Ethylene oxide is used as a fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality ...
and as an accelerator of maturation of tobacco leaves.
Identification of ethylene oxide
Gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substanc ...
is the principal method for analysis and detection of ethylene oxide.pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
derivatives and hydrolysis of ethylene glycol with periodic acid. The produced iodic acid is detected with silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar causti ...
.
Accidents
Ethylene oxide is extremely flammable, and its mixtures with air are explosive. When heated it may rapidly expand, causing fire and explosion. Several industrial accidents have been attributed to ethylene oxide explosion.
The autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature in which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to s ...
is , decomposition temperature of at , minimum inflammable content in the air is 2.7%, and maximum limit is 100%. The NFPA rating is NFPA 704
"NFPA 704: Standard System for the Identification of the Hazards of Materials for Emergency Response" is a standard maintained by the U.S.-based National Fire Protection Association. First "tentatively adopted as a guide" in 1960, and revised sev ...
. Ethylene oxide in presence of water can hydrolyze to ethylene glycol and form polyethylene oxide, which then eventually is oxidized by air and leads to hotspots
Hotspot, Hot Spot or Hot spot may refer to:
Places
* Hot Spot, Kentucky, a community in the United States
Arts, entertainment, and media Fictional entities
* Hot Spot (comics), a name for the DC Comics character Isaiah Crockett
* Hot Spot (Tra ...
that can trigger explosive decomposition.
Fires caused by ethylene oxide are extinguished with conventional media including foam, carbon dioxide or water. Suppression of this activity can be done by blanketing with an inert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to ...
until total pressure reaches the nonexplosive range. Extinguishing of burning ethylene oxide is complicated by its ability to continue burning in an inert atmosphere and in water solutions. Fire suppression is reached only upon dilution with water above 22:1.
La Canonja, Spain accident
On 14 January 2020 in an industrial estate near Tarragona
Tarragona (, ; Phoenician: ''Tarqon''; la, Tarraco) is a port city located in northeast Spain on the Costa Daurada by the Mediterranean Sea. Founded before the fifth century BC, it is the capital of the Province of Tarragona, and part of Tarr ...
, an explosion of an ethoxylation reactor owned by the chemical company Industrias Quimicas de Oxido de Etileno (IQOXE, part of the CL Industrial Group) occurred. The accident launched substantial debris over a radius of about two and a half kilometers, one piece penetrating a distant home and killing an occupant. It is reported that at least three people were killed and seven injured as a direct result of the explosion.
The company was, until the time of the explosion the only producer of ethylene oxide in Spain with an installed capacity of 140,000 tons/year. Half of that production was used to manufacture ethylene glycol for PET production. The accident will be investigated under EU regulations within the context of the European Agency for Safety and Health at Work.
2020 sesame seeds contamination
In September 2020, high levels of pesticides were found in 268 tonnes of sesame
Sesame ( or ; ''Sesamum indicum'') is a flowering plant in the genus ''Sesamum'', also called benne. Numerous wild relatives occur in Africa and a smaller number in India. It is widely naturalized in tropical regions around the world and is cul ...
seeds from India. The contamination had a level of 1000 to 3500 times the limit of 0.05 milligrams per kilogram for ethylene oxide allowed in Europe. This pesticide is forbidden in Europe, it is known to be carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
and mutagenic. A product recall was made, half of the products had an organic certification.
In September, alert was raised by Belgium by RASFF, but the product has also been sold in other EU single market countries such as France and Ireland.
Physiological effects
Effect on microorganisms
Exposure to ethylene oxide gas causes alkylation to microorganisms at a nuclear level. The disinfectant effect of ethylene oxide is similar to that of sterilization by heat, but because of limited penetration, it affects only the surface. ETO sterilization can take up to 12 hours due to its slow action upon microorganisms, and lengthy processing and aeration time.
Effects on humans and animals
Ethylene oxide is an Alkylation, alkylating agent; it has irritating, sensitizing and narcotic effects.[Toxicological Profile For Ethylene Oxide]
Agency for Toxic Substances and Disease Registry, US Public Health Services An increased incidence of peritoneal mesotheliomas was also observed in the animals exposed to concentrations of . Results of human epidemiological studies on workers exposed to ethylene oxide differ. There is evidence from both human and animal studies that inhalation exposure to ethylene oxide can result in a wide range of carcinogenic effects.
Ethylene oxide is toxic by inhalation, with a US Occupational Safety and Health Administration, OSHA permissible exposure limit calculated as a TWA (time weighted average) over 8 hours of 1ppm, and a short term exposure limit (excursion limit) calculated as a TWA over 15 minutes of 5ppm.diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
, a common laboratory solvent of very low toxicity. In view of these insidious properties, continuous electrochemical monitoring is standard practice, and it is forbidden to use ethylene oxide to fumigate building interiors in the European Union, EU and some other jurisdictions.[ The metabolism of ethylene oxide is not completely known. Data from animal studies indicate two possible pathways for the metabolism of ethylene oxide: hydrolysis to ethylene glycol and glutathione conjugation to form mercapturic acid and meththio-metabolites.
Ethylene oxide easily penetrates through ordinary clothing and footwear, causing skin irritation and dermatitis with the formation of blisters, fever and leukocytosis.][Codes]
.
* Eye exposure: /6 hours (rabbit)
* Oral: (rat, median lethal dose, LD50), (rat, Lowest published toxic dose, TDLo), (rat, Toxic dose, TD)
* Inhalation: 12,500 ppm (human, Median lethal dose#Other measures of toxicity, TCLo), 960 ppm/4 hours (dog, median lethal dose, LC50) 33–50 ppm (rat or mouse, TC), 800 ppm/4 hours (rat or mouse, LC50)
* Subcutaneous injection: (cat, LDLo), (mouse, TDLo) (mouse, TD), (rat, LD50).
* Intraperitoneal injection: (mouse, TDLo), (mouse, LD50)
* Intravenous injection: (rabbit, LD50), (mouse, LD50)
* The US Environmental Protection Agency (USEPA) estimated in 2016 that for low doses, the inhalation of ethylene oxide for a lifetime could increase an individual's lifetime cancer risk by as much as 3.0×10−3 per μg/m3 (without considering that early-life exposures are likely more potent). The USEPA estimated the slope of the dose-response declines at higher doses, and extra cancer risk estimates for several occupational exposure scenarios are calculated.
Global demand
Global EO demand has expanded from in 2004 to in 2009, while demand for refined EO expanded from in 2004 to in 2008. In 2009, demand is estimated to have declined to about . Total EO demand registered a growth rate of 5.6% per annum during the period 2005 to 2009 and is projected to grow at 5.7% per annum during 2009 to 2013.
Health and safety regulations
According to Merck Life Science UK 2020 Safety Data Sheet provided to the European Chemicals Agency's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)—a 2006 European Union regulation,Regulation (EC) No 1907/2006
European Parliament. 18 December 2006 ethylene oxide is "presumed to have carcinogenic potential for humans."
References
Cited sources
*
External links
EOSA Promoting the safe use of Ethylene Oxide for SterilizationWebBook page for C2H4ONational Institute for Occupational Safety and Health – Ethylene Oxide Topic Page
{{Authority control
Biocides
Epoxides
Fungicides
Hazardous air pollutants
IARC Group 1 carcinogens
Monomers
Occupational safety and health
Suspected testicular toxicants
Commodity chemicals
Gaseous signaling molecules
Explosive gases
Explosive chemicals
Organic compounds with 2 carbon atoms

 The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol. For comparison, in alcohols the C–O–H angle is about 110°; in
The epoxy cycle of ethylene oxide is an almost regular triangle with bond angles of about 60° and a significant angular strain corresponding to the energy of 105 kJ/mol. For comparison, in alcohols the C–O–H angle is about 110°; in  and more specific reactions are described below.
and more specific reactions are described below.

 Styrene can be obtained in one stage if this reaction is conducted at elevated temperatures () and pressures (), in presence of an aluminosilicate catalyst.
Styrene can be obtained in one stage if this reaction is conducted at elevated temperatures () and pressures (), in presence of an aluminosilicate catalyst.

 The reaction mechanism is as follows:
:
The reaction mechanism is as follows:
:  The dimerization reaction is unselective. By-products include
The dimerization reaction is unselective. By-products include  Reaction of
Reaction of  In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with
In industry, a similar reaction is carried out at high pressure and temperature in the presence of quaternary ammonium or phosphonium salts as a catalyst.
Reaction of ethylene oxide with  Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Catalytic
Substituting formaldehyde by other aldehydes or ketones results in a 2-substituted 1,3-dioxolane (yield: 70–85%, catalyst: tetraethylammonium bromide).
Catalytic  Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.
Oxidation by peroxy acids is efficient for higher alkenes, but not for ethylene. The above reaction is slow and has low yield, therefore it is not used in the industry.

 Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of
Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of