Enolate on:
[Wikipedia]
[Google]
[Amazon]
 In
In

 The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of phenylacetone, deprotonation can produce two different enolates. LDA has been shown to deprotonate the methyl group, which is the kinetic course of the deprotonation. To ensure the production of the kinetic product, a slight excess (1.1 equiv) of lithium diisopropylamide is used, and the ketone is added to the base at −78 °C. Because the ketone is quickly and quantitatively converted to the enolate and base is present in excess at all times, the ketone is unable to act as a proton shuttle to catalyze the gradual formation of the thermodynamic product. A weaker base such as an
The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of phenylacetone, deprotonation can produce two different enolates. LDA has been shown to deprotonate the methyl group, which is the kinetic course of the deprotonation. To ensure the production of the kinetic product, a slight excess (1.1 equiv) of lithium diisopropylamide is used, and the ketone is added to the base at −78 °C. Because the ketone is quickly and quantitatively converted to the enolate and base is present in excess at all times, the ketone is unable to act as a proton shuttle to catalyze the gradual formation of the thermodynamic product. A weaker base such as an 

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, enolates are organic anions derived from the deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
() compounds. Rarely isolated, they are widely used as reagents
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
in the synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
* Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organ ...
of organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s.
Bonding and structure
Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both analkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
and a carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3 ...
.
Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates.

Preparation
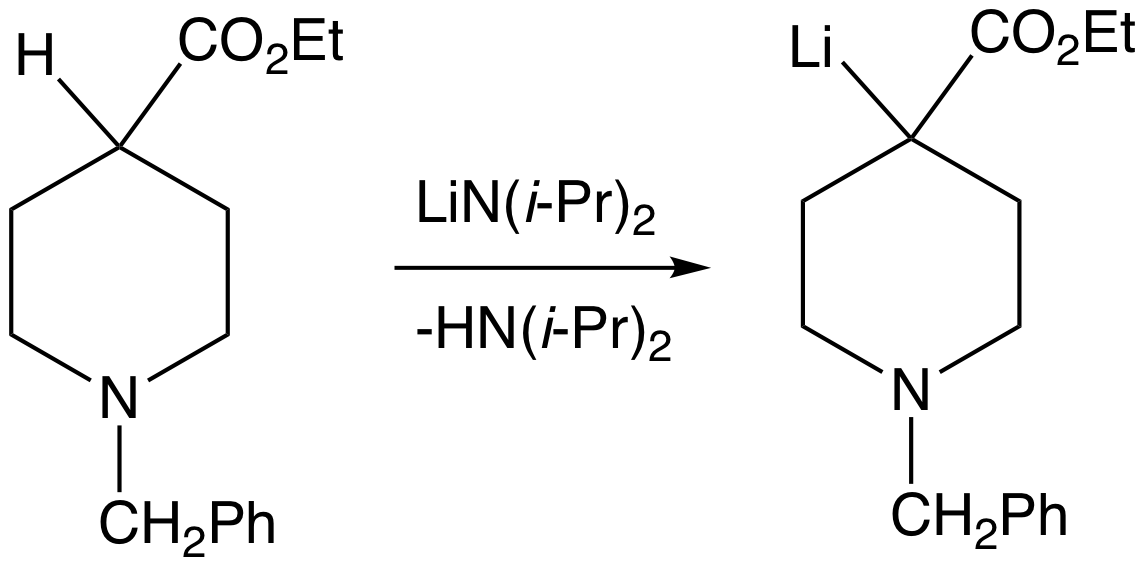
Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from usinglithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
(LDA).
Often, as in conventional Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Ra ...
s, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concentrations, but they still undergo reactions with electrophiles. Many factors affect the behavior of enolates, especially the solvent, additives (e.g. diamines), and the countercation (Li+ vs Na+, etc.). For unsymmetrical ketones, methods exist to control the regiochemistry of the deprotonation.
 The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of phenylacetone, deprotonation can produce two different enolates. LDA has been shown to deprotonate the methyl group, which is the kinetic course of the deprotonation. To ensure the production of the kinetic product, a slight excess (1.1 equiv) of lithium diisopropylamide is used, and the ketone is added to the base at −78 °C. Because the ketone is quickly and quantitatively converted to the enolate and base is present in excess at all times, the ketone is unable to act as a proton shuttle to catalyze the gradual formation of the thermodynamic product. A weaker base such as an
The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. For example, in the case of phenylacetone, deprotonation can produce two different enolates. LDA has been shown to deprotonate the methyl group, which is the kinetic course of the deprotonation. To ensure the production of the kinetic product, a slight excess (1.1 equiv) of lithium diisopropylamide is used, and the ketone is added to the base at −78 °C. Because the ketone is quickly and quantitatively converted to the enolate and base is present in excess at all times, the ketone is unable to act as a proton shuttle to catalyze the gradual formation of the thermodynamic product. A weaker base such as an alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
, which reversibly deprotonates the substrate, affords the more thermodynamically stable benzylic enolate.
Enolates can be trapped by acylation and silylation
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. The process is the basis of organosilicon chemistry.
Of organic compounds
Alcohols, carboxylic acids, amines, thiols, and phosphates can be sil ...
, which occur at oxygen. Silyl enol ethers are common reagents in organic synthesis as illustrated by the Mukaiyama aldol reaction
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate. The reaction was discovered by Teruaki Mukaiyama (1927–2018) in 1973. His choice of reactants allows fo ...
:
Reactions
As powerful nucleophiles, enolates react readily with a variety of electrophiles. These reactions generate new C-C bonds and often new stereocenters. The stereoselectivity and regioselectivity is influenced by additives, solvent,counterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typical ...
s, etc. One important class of electrophiles are alkyl halides, and in this case a classic problem arises: O-alkylation vs C-alkylation. Controlling this selectivity has drawn much attention. The negative charge in enolates is concentrated on the oxygen, but that center is also highly solvated, which leads to C-alkylation.
Other important electrophiles are aldehydes/ketones and Michael acceptors.
:
Aza enolates
Aza enolates (also known as imine anions, enamides, metallated Schiff bases, and metalloenamines) are nitrogen analogous to enolates. When imines get treated with strong bases such as LDA, highly nucleophilic aza enolates are generated. The major benefit of using aza enolates is that they don't undergoself-condensation Self-condensation is an organic reaction in which a chemical compound containing a carbonyl group acts both as the electrophile and the nucleophile in an aldol condensation. It is also called a symmetrical aldol condensation as opposed to a mixed ...
(i.e. aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two ...
for aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
) in a basic or neutral solution, but rather they favor alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
on the alpha-carbon. This is mainly because imines
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
contain carbon-nitrogen double bonds unlike aldehydes, which contain oxygen-carbon double bonds. Since oxygen is more electronegative than nitrogen, it withdraws more electron density from the carbonyl carbon, inducing a greater partially positive charge on the carbon. Therefore, with more electrophilic carbon, aldehydes allow for better nucleophilic addition to the carbon on the carbon-oxygen double bond.
On the other hand, imine has less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
nitrogen which induces a weaker partially positive charge on the carbonyl-carbon. As a result, while imines can still react with organolithiums, they don't react with other nucleophiles (including aza enolates) to undergo nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions d ...
s.
Instead, aza enolates react similarly to enolates, forming SN2 alkylated products. Through nitrogen lone pair conjugation, β-carbon becomes a nucleophilic site, permitting aza enolates to undergo alkylation reactions. Thus, aza enolates can react with numerous electrophiles like epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
s and alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
to form a new carbon-carbon bond on β-carbon.
Two potential reaction mechanisms are shown below:
Since epoxide is a three-membered ring molecule, it has a high degree of ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are ...
. Although the carbons in the ring system are tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
, preferring 109.5 degrees between each atom, epoxide strains the ring angles into 60 degrees. To counter this effect, the nucleophilic aza enolates easily react with epoxides to reduce their ring strains.
Besides reacting with epoxides, aza enolates can also react with alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
(or allyl halides as depicted above) to form a new carbon-carbon sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
. This reaction is one of the key steps in the synthesis of the male aggression pheromone, Oulema melanopus. Aza enolate is generated by LDA reacting with pivaldehyde, which then reacts with an alkyl halide to form an Oulema melanopus intermediate.
Aza enolates can also be formed with Grignard reagents
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
and react with other soft electrophiles, including Michael receptors.
See also
*Nitrile anion Nitrile anions is jargon from the organic product resulting from the deprotonation of alkylnitriles. The proton(s) α to the nitrile group are sufficiently acidic that they undergo deprotonation by strong bases, usually lithium-derived. The produc ...
References
{{Authority control Alcohols Alkene derivatives Reactive intermediates Organic reactions de:Tautomerie#Keto-Enol-Tautomerie