Enhanced weathering on:
[Wikipedia]
[Google]
[Amazon]
Enhanced weathering is a process that aims to accelerate the natural


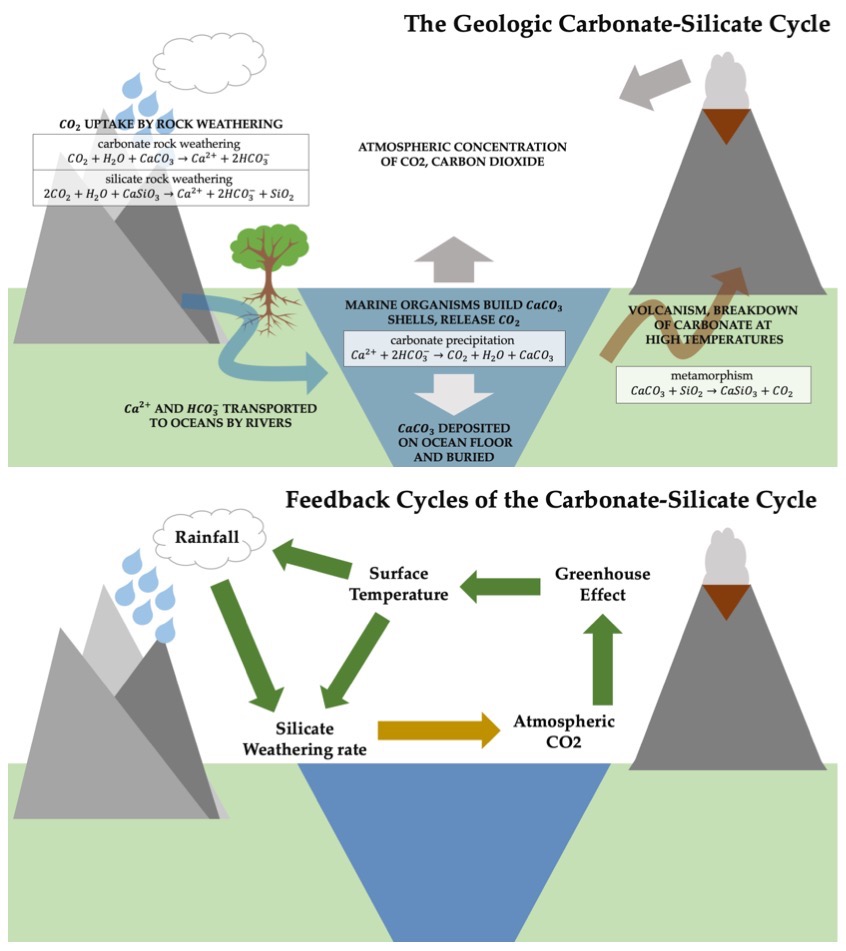 Weathering and biological carbonate precipitation are thought to be only loosely coupled on short time periods (<1000 years). Therefore, an increase in both carbonate and silicate weathering with respect to carbonate precipitation will result in a buildup of alkalinity in the ocean.
Weathering and biological carbonate precipitation are thought to be only loosely coupled on short time periods (<1000 years). Therefore, an increase in both carbonate and silicate weathering with respect to carbonate precipitation will result in a buildup of alkalinity in the ocean.
Enhanced Weathering Conference 2022
Carbon dioxide removal Climate engineering Weathering Enhanced weathering
weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs ''in situ'' (on site, with little or no movement) ...
by spreading finely ground silicate rock, such as basalt
Basalt (; ) is an aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron (mafic lava) exposed at or very near the surface of a rocky planet or moon. More than 90 ...
, onto surfaces which speeds up chemical reactions between rocks, water, and air. It also removes carbon dioxide () from the atmosphere, permanently storing it in solid carbonate mineral
Carbonate minerals are those minerals containing the carbonate ion, .
Carbonate divisions Anhydrous carbonates
*Calcite group: trigonal
** Calcite CaCO3
** Gaspéite (Ni,Mg,Fe2+)CO3
** Magnesite MgCO3
** Otavite CdCO3
**Rhodochrosite MnCO3
** ...
s or ocean alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength ...
. The latter also slows ocean acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxid ...
.
Although existing mine tailings
In mining, tailings are the materials left over after the process of separating the valuable fraction from the uneconomic fraction (gangue) of an ore. Tailings are different to overburden, which is the waste rock or other material that overli ...
or alkaline industrial silicate minerals (such as steel slags, construction & demolition waste, or ash from biomass incineration) may be used at first, mining more basalt might eventually be required to limit climate change.
History
Enhanced weathering has been proposed for both terrestrial and ocean-basedcarbon sequestration
Carbon sequestration is the process of storing carbon in a carbon pool. Carbon dioxide () is naturally captured from the atmosphere through biological, chemical, and physical processes. These changes can be accelerated through changes in lan ...
. Ocean methods are being tested by the non-profit organization Project Vesta to see if they are environmentally and economically viable.
In July 2020, a group of scientists assessed that the geo-engineering technique of enhanced rock weathering, i.e., spreading finely crushed basalt
Basalt (; ) is an aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron (mafic lava) exposed at or very near the surface of a rocky planet or moon. More than 90 ...
on fields – has potential use for carbon dioxide removal by nations, identifying costs, opportunities, and engineering challenges.
Natural mineral weathering and ocean acidification

Weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs ''in situ'' (on site, with little or no movement) ...
is the natural process of rocks and mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
s dissolving to the action of water, ice, acids, salts, plants, animals, and temperature changes. It is mechanical (breaking up rock—also called physical weathering or disaggregation) and chemical (changing the chemical compounds in the rocks). Biological weathering is a form of weathering (mechanical or chemical) by plants, fungi, or other living organisms.
Chemical weathering can happen by different mechanisms, depending mainly on the nature of the minerals involved. This includes solution, hydration, hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
, and oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
weathering. Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
In inorganic ch ...
weathering is a particular type of solution weathering.
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
and silicate mineral
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, silica (silicon dioxide, ) is usually consid ...
s are examples of minerals affected by carbonation weathering. When silicate or carbonate minerals are exposed to rainwater or groundwater, they slowly dissolve due to carbonation weathering: that is the water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
(H2O) and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
() present in the atmosphere form carbonic acid (H2CO3) by the reaction:
:H2O + → H2CO3
This carbonic acid then attacks the mineral to form carbonate ions in solution with the unreacted water. As a result of these two chemical reactions (carbonation and dissolution), minerals, water, and carbon dioxide combine, which alters the chemical composition of minerals and removes from the atmosphere.
In particular, forsterite (a silicate mineral) is dissolved through the reaction:
:Mg2SiO4(s) + 4H2CO3(aq) → 2Mg2+(aq) + 4HCO3−(aq) + H4SiO4(aq)
where "(s)" indicates a substance in a solid state and "(aq)" indicates a substance in an aqueous solution.
Calcite (a carbonate mineral) is instead dissolved through the reaction:
:CaCO3(s) + H2CO3(aq) → Ca2+(aq) + 2HCO3−(aq)
Water with dissolved bicarbonate ions (HCO3−) eventually ends up in the ocean, where the bicarbonate ions are biomineralized to carbonate minerals for shells and skeletons through the reaction:
:Ca2+ + 2HCO3− → CaCO3 + + H2O
The carbonate minerals then eventually sink from the ocean surface to the ocean floor. Most of the carbonate is redissolved in the deep ocean as it sinks.
Over geological time periods these processes are thought to stabilize the Earth's climate
Climate is the long-term weather pattern in an area, typically averaged over 30 years. More rigorously, it is the mean and variability of meteorological variables over a time spanning from months to millions of years. Some of the meteorological ...
. The ratio of carbon dioxide in the atmosphere as a gas (CO2) to the quantity of carbon dioxide converted into carbonate is regulated by a chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
: in case of a change of this equilibrium state, it takes theoretically (if no other alteration is happening during this time) thousands of years to establish a new equilibrium state.
For silicate weathering, the theoretical net effect of dissolution and precipitation is 1 mol of sequestered for every mol of Ca2+ or Mg2+ weathered out of the mineral. Given that some of the dissolved cations react with existing alkalinity in the solution to form CO32− ions, the ratio is not exactly 1:1 in natural systems but is a function of temperature and partial pressure. The net sequestration of carbonate weathering reaction and carbonate precipitation reaction is zero.
 Weathering and biological carbonate precipitation are thought to be only loosely coupled on short time periods (<1000 years). Therefore, an increase in both carbonate and silicate weathering with respect to carbonate precipitation will result in a buildup of alkalinity in the ocean.
Weathering and biological carbonate precipitation are thought to be only loosely coupled on short time periods (<1000 years). Therefore, an increase in both carbonate and silicate weathering with respect to carbonate precipitation will result in a buildup of alkalinity in the ocean.
Terrestrial enhanced weathering
Enhanced weathering was initially used to refer specifically to the spreading of crushed silicate minerals on the land surface. Biological activity in soils has been shown to promote the dissolution of silicate minerals, but there is still uncertainty surrounding how quickly this may happen. Because weathering rate is a function of saturation of the dissolving mineral in solution (decreasing to zero in fully saturated solutions), some have suggested that lack of rainfall may limit terrestrial enhanced weathering, although others suggest that secondary mineral formation or biological uptake may suppress saturation and promote weathering. The amount of energy that is required forcomminution
Comminution is the reduction of solid materials from one average particle size to a smaller average particle size, by crushing, grinding, cutting, vibrating, or other processes. In geology, it occurs naturally during faulting in the upper part of ...
depends on the rate at which the minerals dissolve (less comminution is required for rapid mineral dissolution). A 2012 study suggested a large range in potential cost of enhanced weathering largely due to the uncertainty surrounding mineral dissolution rates.
Oceanic enhanced weathering
To overcome the limitations of solution saturation and to use natural comminution of sand particles from wave energy, silicate minerals may be applied to coastal environments, although the higher pH of seawater may substantially decrease the rate of dissolution, and it is unclear how much comminution is possible from wave action. Alternatively, the direct application of carbonate minerals to theupwelling
Upwelling is an oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrient-rich water from deep water towards the ocean surface. It replaces the warmer and usually nutrient-depleted surface water. The nut ...
regions of the ocean has been investigated. Carbonate minerals are supersaturated in the surface ocean but are undersaturated in the deep ocean. In areas of upwelling, this undersaturated water is brought to the surface. While this technology will likely be cheap, the maximum annual CO2 sequestration potential is limited.
Transforming the carbonate minerals into oxides and spreading this material in the open ocean ('Ocean Liming') has been proposed as an alternative technology. Here the carbonate mineral (CaCO3) is transformed into lime (CaO) through calcination
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), gener ...
. The energy requirements for this technology are substantial.
Mineral carbonation
The enhanced dissolution and carbonation of silicates ( 'mineral carbonation') was first proposed by Seifritz, and developed initially by Lackner et al. and further by the Albany Research Center. This early research investigated the carbonation of extracted and crushed silicates at elevated temperatures (~180 °C) and partial pressures of CO2 (~15 MPa) inside controlled reactors ("ex-situ mineral carbonation"). Some research explores the potential of "in-situ mineral carbonation" in which the CO2 is injected into silicate rock formations to promote carbonate formation underground (see: CarbFix). Mineral carbonation research has largely focused on the sequestration of from flue gas. It could be used for geoengineering if the source of was derived from the atmosphere, e.g. throughdirect air capture
Direct air capture (DAC) is a process of capturing carbon dioxide () directly from the ambient air (as opposed to capturing from point sources, such as a cement factory or biomass power plant) and generating a concentrated stream of for seque ...
or biomass-CCS.
Soil Remineralization
In biogeochemistry, remineralisation (or remineralization) refers to the breakdown or transformation of organic matter (those molecules derived from a biological source) into its simplest inorganic forms. These transformations form a crucial link ...
contributes to the enhanced weathering process. Mixing the soil with crushed rock such as silicate benefits not only plants' health, but also carbon sequestration when calcium or magnesium are present. Remineralize The Earth is a non-profit organization that promotes rock dust applications as natural fertilizers in agriculture fields to restore soils with minerals, improve the quality of vegetation and increase carbon sequestration.
Electrolytic dissolution of silicate minerals
Where abundant electric surplus electricity is available, the electrolytic dissolution of silicate minerals has been proposed and experimentally shown. The process resembles the weathering of some minerals. In addition, hydrogen produced would be a carbon-negative.Cost
In a 2020 techno-economical analysis, the cost of utilizing this method on cropland was estimated at US$80–180 per tonne of CO2. This is comparable with other methods of removing carbon dioxide from the atmosphere currently available (BECCS (US$100–200 per tonne of CO2)-Bio-Energy with Carbon Capture and Storage
Bioenergy with carbon capture and storage (BECCS) is the process of extracting bioenergy from biomass and capturing and storing the carbon, thereby removing it from the atmosphere. The carbon in the biomass comes from the greenhouse gas carbon ...
) and direct air capture
Direct air capture (DAC) is a process of capturing carbon dioxide () directly from the ambient air (as opposed to capturing from point sources, such as a cement factory or biomass power plant) and generating a concentrated stream of for seque ...
and storage at large scale deployment and low-cost energy inputs (US$100–300 per tonne of CO2). In contrast, the cost of reforestation
Reforestation (occasionally, reafforestation) is the natural or intentional restocking of existing forests and woodlands (forestation) that have been depleted, usually through deforestation, but also after clearcutting.
Management
A debat ...
was estimated lower than US$100 per tonne of CO2.
See also
* Olivine#UsesReferences
{{reflistExternal links
Enhanced Weathering Conference 2022
Carbon dioxide removal Climate engineering Weathering Enhanced weathering