Electroless nickel plating on:
[Wikipedia]
[Google]
[Amazon]
 Electroless nickel-phosphorus plating is a
Electroless nickel-phosphorus plating is a
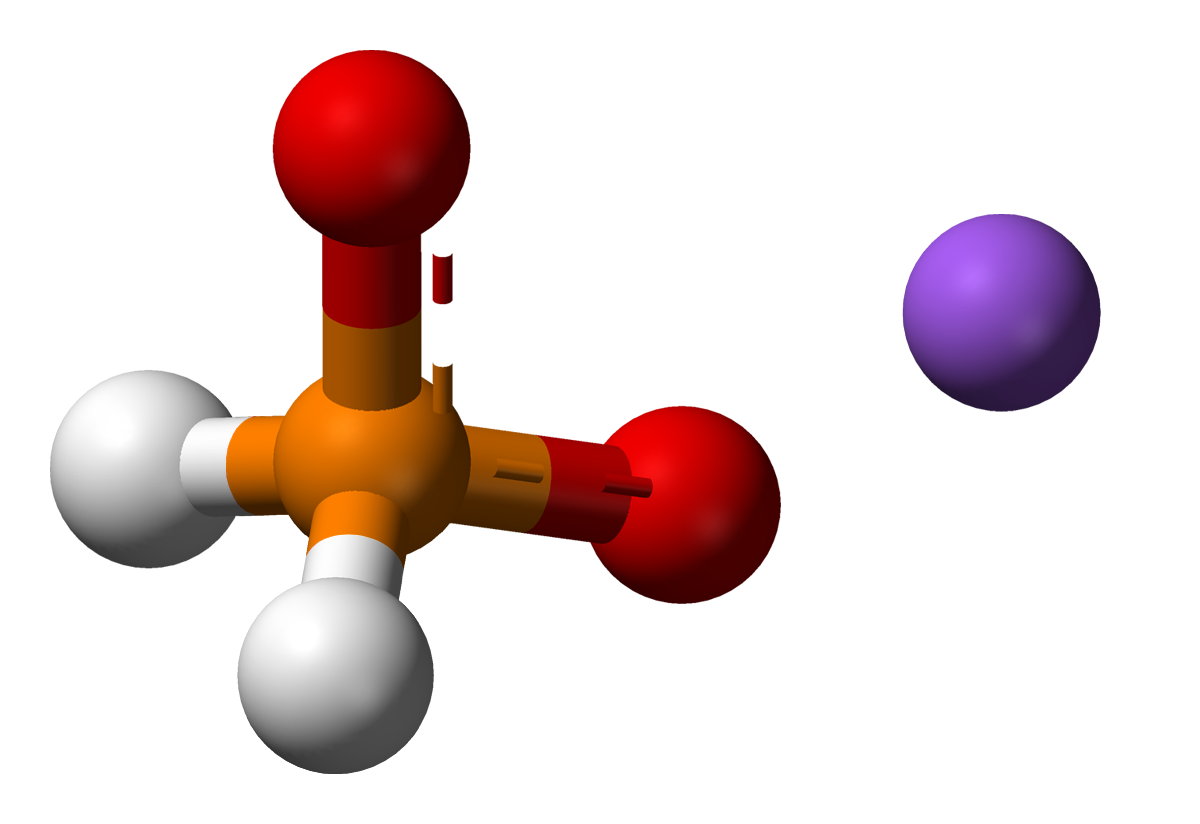 The main ingredients of an electroless nickel plating bath are source of nickel cations , usually nickel sulfate and a suitable reducing agent, such as hypophosphite or
The main ingredients of an electroless nickel plating bath are source of nickel cations , usually nickel sulfate and a suitable reducing agent, such as hypophosphite or
 Electroless nickel-phosphorus is used when wear resistance, hardness and corrosion protection are required. Applications include oilfield valves, rotors, drive shafts, paper handling equipment, fuel rails, optical surfaces for diamond turning, door knobs,
Electroless nickel-phosphorus is used when wear resistance, hardness and corrosion protection are required. Applications include oilfield valves, rotors, drive shafts, paper handling equipment, fuel rails, optical surfaces for diamond turning, door knobs,
ASTM (2009):
ASTM B733 - 04(2009) Standard Specification for Autocatalytic (Electroless) Nickel-Phosphorus Coatings on Metal
. ASTM ():
. Harold Edward Bellis (1969): "Nickel or cobalt wear-resistant compositions and coatings". US Patent 3674447. Granted on 1972-07-04, assigned to
Historical highlights of electroless plating
. ''Plating and Surface Finishing'', volume 71, issue 6, pages 24-27. Thomas Publishing Company (2020):
Pretreatment of Parts for Electroless Nickle Plating
. Online article at the Thomasnet.com website. Accessed on 2020-07-11. Thomas Publishing Company (2020):
The Electro Nickel Plating Process
. Online article at the Thomasnet.com website. Accessed on 2020-07-11. Thomas Publishing Company (2020):
How Electroless Nickel Plating Works
. Online article at the Thomasnet.com website. Accessed on 2020-07-11. =Georgi G. Gavrilov (1979),
Chemical (Electroless) Nickel-Plating
'. Translation by John E. Goodman. Accessed on 2018-09-08. François Auguste Roux (1914):
Process of producing metallic deposits
. US Patent 1207218. Granted 1916-12-05, assigned to L'Aluminium Français, expired on 1933-12-05. M. Bouanani, F. Cherkaoui, R. Fratesi, G. Roventi, and G. Barucca (1999): "Microstructural characterization and corrosion resistance of Ni–Zn–P alloys electrolessly deposited from a sulphate bath". ''Journal of Applied Electrochemistry'', volume 29, pages 637–645. Abner Brenner and Grace E. Riddel (1946):
Nickel plating on steel by chemical reduction
. ''Journal of Research of the National Bureau of Standards'', volume 37, pages 31–34 {{doi, 10.6028/jres.037.019 Abner Brenner and Grace E. Riddel (1946): ''Proc. 33rd Annual Convention of the American Electroplaters' Society'' page 23. Abner Brenner and Grace E. Riddel(1947): ''Proc. 34th Annual Convention of the American Electroplaters' Society'', page 156. Abner Brenner and Grace E. Riddel (1950): "Nickel plating by chemical reduction". US Patent 2532283. Granted on 1950-12-05, expired on 1967-12-05. Abner Brenner (1954): ''Metal Finishing'', volume 52, issue 11, page 68. Abner Brenner (1954): ''Metal Finishing'', volume 52, issue 12, page 61.
Printed circuit board manufacturing
Metal plating
 Electroless nickel-phosphorus plating is a
Electroless nickel-phosphorus plating is a chemical process
In a scientific sense, a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds. Such a chemical process can occur by itself or be caused by an outside force, and involves a chemical reaction of some ...
that deposits an even layer of nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
-phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
on the surface of a solid substrate, like metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
or plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adapta ...
. The process involves dipping the substrate in a water solution containing nickel salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
and a phosphorus-containing reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth met ...
, usually a hypophosphite salt. It is the most common version of electroless nickel plating (EN plating) and is often referred by that name. A similar process uses a borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihyd ...
reducing agent, yielding a nickel-boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
coating instead.
Unlike electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
, electroless plating
Electroless plating, also known as chemical plating or autocatalytic plating, is a class of industrial chemical processes that create metal coatings on various materials by autocatalytic chemical reduction of metal cations in a liquid bath. This ...
processes in general do not require passing an electric current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The movi ...
through the bath and the substrate; the reduction of the metal cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s in solution to metallic is achieved by purely chemical means, through an autocatalytic reaction. Thus electroless plating creates an even layer of metal regardless of the geometry of the surface – in contrast to electroplating which suffers from uneven current density
In electromagnetism, current density is the amount of charge per unit time that flows through a unit area of a chosen cross section. The current density vector is defined as a vector whose magnitude is the electric current per cross-sectional a ...
due to the effect of substrate shape on the electric resistance of the bath and therefore on the current distribution within it. Moreover, electroless plating can be applied to non-conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. Electric current is gene ...
surfaces.
Electroless plating has many industrial applications, from merely decorative to the prevention of corrosion and wear. It can be used to apply composite
Composite or compositing may refer to:
Materials
* Composite material, a material that is made from several different substances
** Metal matrix composite, composed of metal and other parts
** Cermet, a composite of ceramic and metallic materials ...
coatings, by suspending suitable powders in the bath.
Historical overview
The reduction of nickel salts to nickel metal by hypophosphite was accidentally discovered by Charles Adolphe Wurtz in 1844. In 1911,François Auguste Roux
François () is a French masculine given name and surname, equivalent to the English name Francis.
People with the given name
* Francis I of France, King of France (), known as "the Father and Restorer of Letters"
* Francis II of France, King ...
of L'Aluminium Français patented the process (using both hypophosphite and orthophosphite) for general metal plating.
However, Roux's invention does not seem to have received much commercial use. In 1946 the process was accidentally rediscovered by Abner Brenner
In the Hebrew Bible, Abner ( he, אַבְנֵר ) was the cousin of King Saul and the commander-in-chief of his army. His name also appears as "Abiner son of Ner", where the longer form Abiner means "my father is Ner".
Biblical narrative
A ...
and Grace E. Riddell
Grace may refer to:
Places United States
* Grace, Idaho, a city
* Grace (CTA station), Chicago Transit Authority's Howard Line, Illinois
* Little Goose Creek (Kentucky), location of Grace post office
* Grace, Carroll County, Missouri, an uninco ...
of the National Bureau of Standards
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical sci ...
. They tried adding various reducing agents to an electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be ...
bath in order to prevent undesirable oxidation reactions at the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
. When they added sodium hypophosphite, they observed that the amount of nickel that was deposited at the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
exceeded the theoretical limit of Faraday's law.
Brenner and Riddel presented their discovery at the 1946 Convention of the American Electroplaters' Society
American(s) may refer to:
* American, something of, from, or related to the United States of America, commonly known as the "United States" or "America"
** Americans, citizens and nationals of the United States of America
** American ancestry, pe ...
(AES); a year later, at the same conference they proposed the term "electroless" for the process and described optimized bath formulations, that resulted in a patent.
A declassified US Army technical report in 1963 credits the discovery to Wurtz and Roux more than to Brenner and Riddell.
During 1954–1959, a team led by Gregorie Gutzeit at General American Transportation Corporation
GATX Corporation is a railcar lessor that owns fleets in North America, Europe, and Asia. In addition, jointly with Rolls-Royce Limited, it owns one of the largest aircraft spare engine lease portfolios. It is headquartered in Chicago, Illinois. ...
greatly developed the process, determining the optimum parameters and concentrations of the bath, and introducing many important additives to speed up the deposition rate and prevent unwanted reactions, such as spontaneous deposition. They also studied the chemistry of the process.
In 1969, Harold Edward Bellis from DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in ...
filed a patent for a general class of electroless plating processes using sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula Na BH4. This white solid, usually encountered as an aqueous basic solution, is a reducing agent that finds applica ...
, dimethylamine borane, or sodium hypophosphite, in the presence of thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
salts, thus producing a metal-thallium-boron or metal-thalium-phosphorus; where the metal could be either nickel or cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
. The boron or phosphorus contents was claimed to be variable from 0.1 to 12%, and that of thallium from 0.5 to 6%. The coatings were claimed to be "an intimate dispersion of hard trinickel boride
Trinickel boride is a compound of nickel and boron with chemical formula . It is one of the nickel boride (disambiguation), borides of nickel.
The compound was described in 1959 by R. Fruchart, S. Rundquist, and L. H. Anderson and R. Kiessling. ...
() or nickel phosphide () in a soft matrix of nickel and thallium".
Procedure
Surface cleaning
Before plating, the surface of the material must be thoroughly cleaned. Unwanted solids left on the surface cause poor plating. Cleaning is usually achieved by a series of chemical baths, including non-polarsolvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s to remove oils and greases, as well as acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
s and alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
s to remove oxides, insoluble organics, and other surface contaminants. After applying each bath, the surface must be thoroughly rinsed with water to remove any residue of the cleaning chemicals.
Internal stresses in the substrate created by machining or welding can affect the plating.
Plating bath
 The main ingredients of an electroless nickel plating bath are source of nickel cations , usually nickel sulfate and a suitable reducing agent, such as hypophosphite or
The main ingredients of an electroless nickel plating bath are source of nickel cations , usually nickel sulfate and a suitable reducing agent, such as hypophosphite or borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihyd ...
.
With hypophosphite, the main reaction that produces the nickel plating yields orthophosphite , elemental phosphorus, protons and molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
:
: 2 + 8 + 2 → 2 (s) + 6 + 2 + 2 (s) + 3 (g)
This reaction is catalyzed by some metals including cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
, rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
, and nickel itself. Because of the latter, the reaction is auto-catalytic, and proceeds spontaneously once an initial layer of nickel has formed on the surface.
The plating bath also often includes:
* complexing agents, such as carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
s or amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
s to increase phosphate solubility and to prevent the white-out phenomena by slowing the reaction.
* stabilizers, such as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
salts, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
compounds, or various organic compounds, to slow the reduction by co-depositing with the nickel.
* buffers, to maintain the acidity of the bath. Many complexing agents act as buffers.
* brighteners, such as cadmium
Cadmium is a chemical element with the Symbol (chemistry), symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Li ...
salts or certain organic compounds, to improve the surface finish. They are mostly co-deposited with nickel (like the stabilizers).
* surfactants, to keep the deposited layer hydrophilic in order to reduce pitting and staining.
* accelerators, such as certain sulfur compounds, to counteract the reduction of plating rate caused by complexing agents. They are usually co-deposited and may cause discoloration.
Surface activation
Because of the autocatalytic character of the reaction, the surface to be plated must be activated by making it hydrophilic, then ensuring that it consists of a metal with catalytic activity. If the substrate is not made of one of those metals, then a thin layer of one of them must be deposited first, by some other process. If the substrate is a metal that is moreelectropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
than nickel, such as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
and aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, an initial nickel film will be created spontaneously by a redox reaction with the bath, such as:
: (s) + (aq) → (s) + (aq)
: 2 (s) + 3 (aq) → 3 (s) + 2 (aq)
For metals that are less electropositive than nickel, such as copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
, the initial nickel layer can be created by immersing a piece of a more electropositive metal, such as zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, electrically connected to the substrate, thus creating a shorted Galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus ...
.
On substrates that are not metallic but are electrically conductive, such as graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
, the initial layer can be created by briefly running an electrical current through it and the bath, as in electroplating. If the substrate is not conductive, such as ABS and other plastics, one can use an activating bath containing a noble metal
A noble metal is ordinarily regarded as a metallic chemical element that is generally resistant to corrosion and is usually found in nature in its raw form. Gold, platinum, and the other platinum group metals ( ruthenium, rhodium, palladium, o ...
salt, like palladium chloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value ...
or silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar causti ...
, and a suitable reducing agent.
Activation is done with a weak acid etch, nickel strike, or a proprietary solution, if the substrate is non-metallic.
After-plating treatment
After plating, an anti-oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
or anti- tarnish chemical coating, such as phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
or chromate, is applied, followed by rinsing with water and dried to prevent staining. Baking may be necessary to improve the hardness and adhesion of the plating, anneal any internal stresses, and expel trapped hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
that may make it brittle.
Variants
The processes for electroless nickel-phosphorus plating can be modified by substitutingcobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
for nickel, wholly or partially, with relatively little changes. Other nickel-phosphorus alloys can be created with suitable baths, such as nickel-zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
-phosphorus.
Composites by codeposition
Electroless nickel-phosphorus plating can producecomposite material
A composite material (also called a composition material or shortened to composite, which is the common name) is a material which is produced from two or more constituent materials. These constituent materials have notably dissimilar chemical or ...
s consisting of minute solid particles embedded in the nickel-phosphorus coat. The general procedure is to suspend the particles in the plating bath, so that the growing metal layer will surround and cover them. This procedure was initially developed by Odekerken in 1966 for electrodeposited nickel-chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
coatings. In that study, in an intermediate layer, finely powdered particles, like aluminum oxide and polyvinyl chloride (PVC) resin, were distributed within a metallic matrix. By changing the baths, the procedure can create coatings with multiple layers of different composition.
The first commercial application of their work was electroless nickel-silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal s ...
coatings on the Wankel internal combustion engine. Another commercial composite in 1981 incorporated polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemo ...
(nickel-phosphorus PTFE). However, the co-deposition of diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
and PTFE particles was more difficult than that of aluminum oxide or silicon carbide. The feasibility to incorporate the second phase of fine particles, the size of a nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, ...
to micrometer Micrometer can mean:
* Micrometer (device), used for accurate measurements by means of a calibrated screw
* American spelling of micrometre
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; ...
, within a metal-alloy matrix has initiated a new generation of composite coatings.
Characteristics
Advantages and disadvantages
Compared to the electrolytic process, a major advantage of electroless nickel plating is that it creates an even coating of a desired thickness and volume, even in parts with complex shape, recesses, and blind holes. Because of this property, it may often be the only option. Another major advantage of EN plating is that it does not require electrical power, electrical apparatuses, or sophisticated jigs and racks. If properly formulated, EN plating may also provide a less porous coating, harder and more resistant tocorrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
and hydrogen absorption.
Electroless nickel plating also can produce coatings that are free of built-in mechanical stress, or even have compressive stress.
A disadvantage is the higher cost of the chemicals, which are consumed in proportion to the mass of nickel deposited; whereas in electroplating the nickel ions are replenished by the metallic nickel anode. Automatic mechanisms may be needed to replenish those reagents during plating.
The specific characteristics vary depending on the type of EN plating and nickel alloy used, which are chosen to suit the application.
Types
The metallurgical properties of the alloy depend on the percentage of phosphorus. * Low-phosphorus coatings have up to 4% P contents. Their hardness reaches up to 60 on the Rockwell C scale. * Medium-phosphorus coatings, the most common type, are defined as those with 4 to 10% P, although the range depends on the application: up to 4–7% for decorative applications, 6–9% for industrial applications, and 4–10% for electronics. * High-phosphorus coatings have 10–14% P. They are preferred for parts that will be exposed to highly corrosive acidic environments such as oil drilling and coal mining. Their hardness mat score up to 600 on Vickers test.Surface finish
Electroless nickel plating can have a matte, semi-bright, or bright finish.Structure
Electroless nickel-phosphorus coatings with less than 7% phosphorus are solid solutions with a microcrystalline structure, with each grain 2–6 nm across. Coatings with more than 10% phosphorus areamorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language, Gr ...
. Between these two limits, the coating is a mixture of amorphous and microcrystalline materials.
Physical properties
The melting point of the nickel-phosphorus alloy deposited by the EN process is significantly lower than that of pure nickel (1445 °C), and decreases as the phosphorus content increases, down to 890 °C at about 14% P. The magnetic properties of the coatings decrease with increasing phosphorus contents. Coatings with more than 11.2% P are non-magnetic. Solderability of low-phosphorus coatings is good, but decreases with increasing P contents.Porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measur ...
decreases as the phosphorus contents increases, while hardness, wear resistance, and resistance to corrosion increase.
Applications
 Electroless nickel-phosphorus is used when wear resistance, hardness and corrosion protection are required. Applications include oilfield valves, rotors, drive shafts, paper handling equipment, fuel rails, optical surfaces for diamond turning, door knobs,
Electroless nickel-phosphorus is used when wear resistance, hardness and corrosion protection are required. Applications include oilfield valves, rotors, drive shafts, paper handling equipment, fuel rails, optical surfaces for diamond turning, door knobs, kitchen utensil
A kitchen utensil is a small hand held tool used for food preparation. Common kitchen tasks include cutting food items to size, heating food on an open fire or on a stove, baking, grinding, mixing, blending, and measuring; different utensils a ...
s, bathroom fixtures, electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described ...
/mechanical
Mechanical may refer to:
Machine
* Machine (mechanical), a system of mechanisms that shape the actuator input to achieve a specific application of output forces and movement
* Mechanical calculator, a device used to perform the basic operations ...
tools and office equipment.
Due to the high hardness of the coating, it can be used to salvage worn parts. Coatings of 25 to 100 micrometers can be applied and machined back to the final dimensions. Its uniform deposition profile means it can be applied to complex components not readily suited to other hard-wearing coatings like hard chromium.
It is also used extensively in the manufacture of hard disk drive
A hard disk drive (HDD), hard disk, hard drive, or fixed disk is an electro-mechanical data storage device that stores and retrieves digital data using magnetic storage with one or more rigid rapidly rotating platters coated with mag ...
s, as a way of providing an atomically smooth coating to the aluminium disks. The magnetic layers are then deposited on top of this film, usually by sputtering and finishing with protective carbon and lubrication layers.
Its use in the automotive industry for wear resistance has increased significantly. However, it is important to recognize that only End of Life Vehicles Directive or RoHS
The Restriction of Hazardous Substances Directive 2002/95/EC (RoHS 1), short for Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, was adopted in February 2003 by the European Uni ...
compliant process types (free from heavy metal stabilizers) may be used for these applications.
Printed circuit boards
Elecroless nickel plating, covered by a thin layer ofgold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
, is used in the manufacture of printed circuit board
A printed circuit board (PCB; also printed wiring board or PWB) is a medium used in electrical and electronic engineering to connect electronic components to one another in a controlled manner. It takes the form of a laminated sandwich str ...
s (PCBs), to avoid oxidation and improving the solderability of copper contacts and plated through holes and vias. The gold is typically applied by quick immersion in a solution containing gold salts. This process is known in the industry as electroless nickel immersion gold (ENIG). A variant of this process adds a thin layer of electroless palladium over the nickel, a process known by the acronym ENEPIG.
Standards
* AMS-2404 * AMS-C-26074 *ASTM
ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, an ...
B-733
* ASTM-B-656 (inactive)
* Mil-C-26074E
* MIL-DTL-32119
* IPC-4552 (for ENIG)
* IPC-7095 (for ENIG)
See also
* Nickel electroplating *Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that deter ...
* Organic Solderability Preservative (OSP)
* Electroless nickel-boron plating
Electroless nickel-boron coating (often called NiB coating) is a metal plating process that can create a layer of a nickel-boron alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a wate ...
* Electroless copper plating Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing copper salts and a reducing ...
References
ASTM B733 - 04(2009) Standard Specification for Autocatalytic (Electroless) Nickel-Phosphorus Coatings on Metal
. ASTM ():
. Harold Edward Bellis (1969): "Nickel or cobalt wear-resistant compositions and coatings". US Patent 3674447. Granted on 1972-07-04, assigned to
DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in ...
, expired 1989-07-04
G. O. Mallory and J. B. Hajdu, editors (1990): ''Electroless plating: fundamentals and applications''. 539 pages.
Charles R. Shipley Jr. (1984):Historical highlights of electroless plating
. ''Plating and Surface Finishing'', volume 71, issue 6, pages 24-27. Thomas Publishing Company (2020):
Pretreatment of Parts for Electroless Nickle Plating
. Online article at the Thomasnet.com website. Accessed on 2020-07-11. Thomas Publishing Company (2020):
The Electro Nickel Plating Process
. Online article at the Thomasnet.com website. Accessed on 2020-07-11. Thomas Publishing Company (2020):
How Electroless Nickel Plating Works
. Online article at the Thomasnet.com website. Accessed on 2020-07-11. =Georgi G. Gavrilov (1979),
Chemical (Electroless) Nickel-Plating
'. Translation by John E. Goodman. Accessed on 2018-09-08. François Auguste Roux (1914):
Process of producing metallic deposits
. US Patent 1207218. Granted 1916-12-05, assigned to L'Aluminium Français, expired on 1933-12-05. M. Bouanani, F. Cherkaoui, R. Fratesi, G. Roventi, and G. Barucca (1999): "Microstructural characterization and corrosion resistance of Ni–Zn–P alloys electrolessly deposited from a sulphate bath". ''Journal of Applied Electrochemistry'', volume 29, pages 637–645. Abner Brenner and Grace E. Riddel (1946):
Nickel plating on steel by chemical reduction
. ''Journal of Research of the National Bureau of Standards'', volume 37, pages 31–34 {{doi, 10.6028/jres.037.019 Abner Brenner and Grace E. Riddel (1946): ''Proc. 33rd Annual Convention of the American Electroplaters' Society'' page 23. Abner Brenner and Grace E. Riddel(1947): ''Proc. 34th Annual Convention of the American Electroplaters' Society'', page 156. Abner Brenner and Grace E. Riddel (1950): "Nickel plating by chemical reduction". US Patent 2532283. Granted on 1950-12-05, expired on 1967-12-05. Abner Brenner (1954): ''Metal Finishing'', volume 52, issue 11, page 68. Abner Brenner (1954): ''Metal Finishing'', volume 52, issue 12, page 61.