E919 on:
[Wikipedia]
[Google]
[Amazon]
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after
 It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example,
It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example,
William A. Tilden
Sir William Augustus Tilden (15 August 1842 – 11 December 1926) was a British chemist. He discovered that isoprene could be made from turpentine. He was unable to turn this discovery into a way to make commercially viable synthetic rubber.
L ...
, who was the first to produce it as a pure compound.
Structure and synthesis
The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°.Production
Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine andnitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
; This reaction reverses above 100 °C.
: Cl2 + 2 NO → 2 NOCl
* By reduction of nitrogen dioxide with hydrogen chloride:
: 2NO2 + 4 HCl → 2NOCl + 2H2O + Cl2
Occurrence in aqua regia
NOCl also arises from the combination of hydrochloric and nitric acids according to the following reaction: :HNO3 + 3 HCl → 2 l+ 2 H2O + NOCl In nitric acid, NOCl is readily oxidized into nitrogen dioxide. The presence of NOCl in aqua regia was described by Edmund Davy in 1831.Reactions
NOCl behaves as an electrophile and an oxidant in most of its reactions. Withhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
acceptors, for example antimony pentachloride, converts to nitrosonium
The nitrosonium ion is , in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obta ...
salts:
: NOCl + SbCl5 → Osup>+ bCl6sup>−
In a related reaction, sulfuric acid gives nitrosylsulfuric acid, the mixed acid anhydride of nitrous and sulfuric acid:
: ClNO + H2SO4 → ONHSO4 + HCl
NOCl reacts with silver thiocyanate to give silver chloride and the pseudohalogen nitrosyl thiocyanate:
: ClNO + AgSCN → AgCl + ONSCN
Similarly, it reacts with silver cyanide to give nitrosyl cyanide.
Nitrosyl chloride is used to prepare metal nitrosyl complex
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat hypertension.
Metal nitrosyl complexes are complex (chemistry), complexes that contain nitric oxide, NO, bonded to a transition me ...
es. With molybdenum hexacarbonyl, NOCl gives the dinitrosyldichloride complex:
:Mo(CO)6 + 2 NOCl → MoCl2(NO)2 + 6 CO
It dissolves platinum:
:Pt + 6 NOCl → (NO+)2 tCl6sup>2- + 4 NO
Applications in organic synthesis
Aside from its role in the production of caprolactam, NOCl finds some other uses inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. It adds to alkenes to afford α-chloro oximes. The addition of NOCl follows the Markovnikov rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a p ...
. Ketenes also add NOCl, giving nitrosyl derivatives:
: H2C=C=O + NOCl → ONCH2C(O)Cl
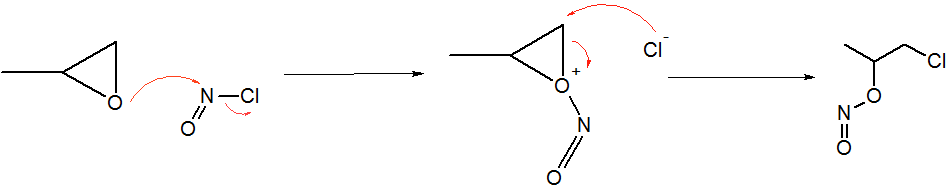
Epoxides react with NOCl to give an α-chloronitritoalkyl derivatives. In the case of propylene oxide, the addition proceeds with high regiochemistry:
: It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example,
It converts amides to ''N''-nitroso derivatives. NOCl converts some cyclic amines to the alkenes. For example, aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivati ...
reacts with NOCl to give ethene, nitrous oxide and hydrogen chloride.
Industrial applications
NOCl andcyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
react photochemically to give cyclohexanone oxime hydrochloride. This process exploits the tendency of NOCl to undergo photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
into NO and Cl radicals. The cyclohexanone oxime is converted to caprolactam, a precursor to nylon-6.
Safety
Nitrosyl chloride is very toxic and irritating to the lungs, eyes, and skin.References
External links
*{{Commonscatinline Chloride Oxychlorides Nitrogen(III) compounds Nitrogen oxohalides Gases with color