E640 on:
[Wikipedia]
[Google]
[Amazon]
Glycine (symbol Gly or G; ) is an
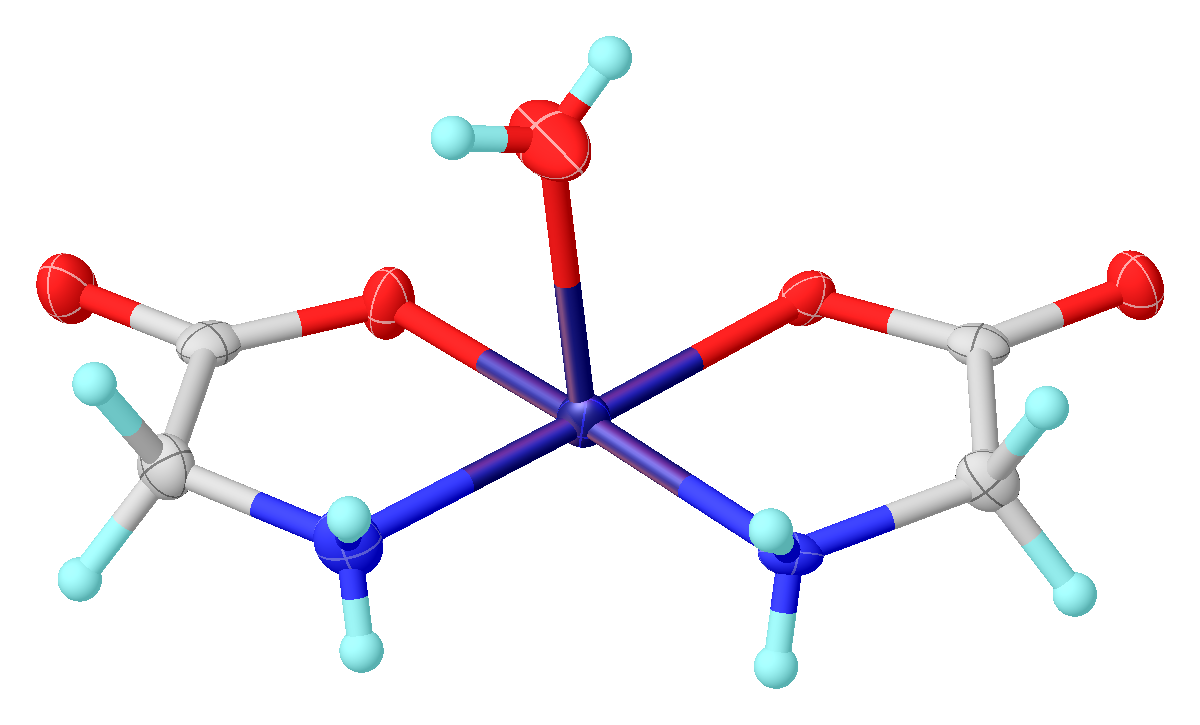
 Glycine functions as a bidentate ligand for many metal ions, forming
Glycine functions as a bidentate ligand for many metal ions, forming
 Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of
Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of
Glycine MS Spectrum
* *
{{Authority control Flavor enhancers Glucogenic amino acids Inhibitory amino acids Proteinogenic amino acids Glycine receptor agonists NMDA receptor agonists E-number additives Pages including recorded pronunciations
amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
that has a single hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom as its side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
. It is the simplest stable amino acid (carbamic acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields an equ ...
is unstable), with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino aci ...
s. It is encoded
In communications and information processing, code is a system of rules to convert information—such as a letter, word, sound, image, or gesture—into another form, sometimes shortened or secret, for communication through a communication ...
by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
in secondary protein structure
Protein secondary structure is the three dimensional form of ''local segments'' of proteins. The two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary structure ...
due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a ''Clostridium tetani
''Clostridium tetani'' is a common soil bacterium and the causative agent of tetanus. Vegetative cells of ''Clostridium tetani'' are usually rod-shaped and up to 2.5 μm long, but they become enlarged and tennis racket- or drumstick-shaped wh ...
'' infection) can cause spastic paralysis due to uninhibited muscle contraction.
It is the only achiral proteinogenic amino acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino aci ...
. It can fit into hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are ...
or hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
environments, due to its minimal side chain of only one hydrogen atom.
History and etymology
Glycine was discovered in 1820 by the French chemistHenri Braconnot
Henri Braconnot (29 May 1780 – 13 January 1855) was a French chemist and pharmacist.
He was born in Commercy, his father being a counsel at the local parliament. At the death of his father, in 1787, Henri began his instruction in an elementar ...
when he hydrolyzed gelatin by boiling it with sulfuric acid. He originally called it "sugar of gelatin", but the French chemist Jean-Baptiste Boussingault showed that it contained nitrogen. The American scientist Eben Norton Horsford
Eben Norton Horsford (27 July 1818 – 1 January 1893) was an American scientist who taught agricultural chemistry in the Lawrence Scientific School at Harvard from 1847 to 1863. Later he was known for his reformulation of baking powder, his int ...
, then a student of the German chemist Justus von Liebig, proposed the name "glycocoll"; however, the Swedish
Swedish or ' may refer to:
Anything from or related to Sweden, a country in Northern Europe. Or, specifically:
* Swedish language, a North Germanic language spoken primarily in Sweden and Finland
** Swedish alphabet, the official alphabet used by ...
chemist Berzelius suggested the simpler name "glycine". The name comes from the Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
word γλυκύς "sweet tasting" (which is also related to the prefixes '' glyco-'' and '' gluco-'', as in '' glycoprotein'' and ''glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
''). In 1858, the French chemist Auguste Cahours determined that glycine was an amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
of acetic acid.
Production
Although glycine can be isolated from hydrolyzed protein, this route is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis. The two main processes are amination ofchloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds a ...
with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
, giving glycine and ammonium chloride, and the Strecker amino acid synthesis, which is the main synthetic method in the United States and Japan. About 15 thousand tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s are produced annually in this way.
Glycine is also cogenerated as an impurity in the synthesis of EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
, arising from reactions of the ammonia coproduct.
Chemical reactions
Its acid–base properties are most important. In aqueous solution, glycine isamphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
One type of amphoteric species are amphipro ...
: below pH = 2.4, it converts to the ammonium cation called glycinium. Above about 9.6, it converts to glycinate.
: Glycine functions as a bidentate ligand for many metal ions, forming
Glycine functions as a bidentate ligand for many metal ions, forming amino acid complex Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the trans ...
es. A typical complex is Cu(glycinate)2, i.e. Cu(H2NCH2CO2)2, which exists both in cis and trans isomers.
With acid chlorides, glycine converts to the amidocarboxylic acid, such as hippuric acid
Hippuric acid ( Gr. ''hippos'', horse, ''ouron'', urine) is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenol ...
and acetylglycine. With nitrous acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in solution, in the gas phase and in the form of nitrite () salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagent ...
, one obtains glycolic acid
Glycolic acid (or hydroxyacetic acid; chemical formula HOCH2CO2H) is a colorless, odorless and hygroscopic crystalline solid, highly soluble in water. It is used in various skin-care products. Glycolic acid is widespread in nature. A glycolate (s ...
(van Slyke determination The Van Slyke determination is a chemical test for the determination of amino acids containing a primary amine group. It is named after the biochemist Donald Dexter Van Slyke (1883-1971).
One of Van Slyke's first professional achievements was the ...
). With methyl iodide, the amine becomes quaternized to give trimethylglycine
Trimethylglycine is an amino acid derivative that occurs in plants. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because, in the 19th century, it was discovered in sugar beets. It has a sweet and umami ...
, a natural product:
: + 3 CH3I → + 3 HI
Glycine condenses with itself to give peptides, beginning with the formation of glycylglycine
Glycylglycine is the dipeptide of glycine, making it the simplest peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are call ...
:
:2 → + H2O
Pyrolysis of glycine or glycylglycine gives 2,5-diketopiperazine, the cyclic diamide.
It forms esters with alcohols. They are often isolated as their hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative n ...
, e.g., glycine methyl ester hydrochloride
Glycine methyl ester hydrochloride is the organic compound with the formula H3O2CCH2NH3l. A white, water-soluble solid, it is the hydrochloride of the methyl ester of the amino acid glycine.
Synthesis and reactions
Glycine methyl ester hydrochlo ...
. Otherwise the free ester tends to convert to diketopiperazine
A diketopiperazine (DKP), also known as a ''dioxopiperazine'' or ''piperazinedione'', is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, ...
.
As a bifunctional molecule, glycine reacts with many reagents. These can be classified into N-centered and carboxylate-center reactions.
Metabolism
Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The ...
, but the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. In most organisms, the enzyme serine hydroxymethyltransferase
Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme () which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine ...
catalyses this transformation via the cofactor pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent a ...
:
: serine + tetrahydrofolate
Tetrahydrofolic acid (THFA), or tetrahydrofolate, is a folic acid derivative.
Metabolism
Human synthesis
Tetrahydrofolic acid is produced from dihydrofolic acid by dihydrofolate reductase. This reaction is inhibited by methotrexate.
It is co ...
→ glycine + ''N5'',''N10''-methylene tetrahydrofolate + H2O
In the liver of vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () (chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, with c ...
s, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:
: CO2 + NH + ''N5'',''N10''-methylene tetrahydrofolate + NADH + H+ ⇌ Glycine + tetrahydrofolate + NAD+
In addition to being synthesized from serine, glycine can also be derived from threonine, choline Choline is an essential nutrient for humans and many other animals. Choline occurs as a cation that forms various salts (X− in the depicted formula is an undefined counteranion). Humans are capable of some ''de novo synthesis'' of choline but r ...
or hydroxyproline via inter-organ metabolism of the liver and kidneys.
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants is the reverse of the glycine synthase pathway mentioned above. In this context, the enzyme system involved is usually called theglycine cleavage system
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine. The same set of enzymes is sometimes r ...
:
: Glycine + tetrahydrofolate + NAD+ ⇌ CO2 + NH + ''N5'',''N10''-methylene tetrahydrofolate + NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an aden ...
+ H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the ...
.
In the third pathway of its degradation, glycine is converted to glyoxylate
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Str ...
by D-amino acid oxidase
D-amino acid oxidase (DAAO; also OXDA, DAMOX) is an enzyme with the function on a molecular level to oxidize D-amino acids to the corresponding α-keto acids, producing ammonia and hydrogen peroxide. This results in a number of physiological e ...
. Glyoxylate is then oxidized by hepatic lactate dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one ...
to oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
in an NAD+-dependent reaction.
The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life varied between 0.5 and 4.0 hours.
Glycine is extremely sensitive to antibiotics which target folate, and blood glycine levels drop severely within a minute of antibiotic injections. Some antibiotics can deplete more than 90% of glycine within a few minutes of being administered.
Physiological function
The principal function of glycine is it acts as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine due to its periodically repeated role in the formation of collagen's helix structure in conjunction withhydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank.
Structure and discovery
In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelati ...
. In the genetic code
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
, glycine is coded by all codons starting with GG, namely GGU, GGC, GGA and GGG.
As a biosynthetic intermediate
In higher eukaryotes,δ-aminolevulinic acid
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in ...
, the key precursor to porphyrins
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compo ...
, is biosynthesized from glycine and succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A.
Sources
It is an important intermediate in the citric acid cycle, where it is synthesized from α-ketoglutarate by α-ketoglutarate d ...
by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines ...
s.
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in thecentral nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all p ...
, especially in the spinal cord
The spinal cord is a long, thin, tubular structure made up of nervous tissue, which extends from the medulla oblongata in the brainstem to the lumbar region of the vertebral column (backbone). The backbone encloses the central canal of the sp ...
, brainstem, and retina
The retina (from la, rete "net") is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then ...
. When glycine receptors
The glycine receptor (abbreviated as GlyR or GLR) is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory ...
are activated, chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
enters the neuron via ionotropic receptors, causing an inhibitory postsynaptic potential
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential.Purves et al. Neuroscience. 4th ed. Sunderland (MA): Sinauer Associates, Incorporated; 2008. ...
(IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA
''N''-methyl--aspartic acid or ''N''-methyl--aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike ...
) glutamatergic
Glutamatergic means "related to glutamate". A glutamatergic agent (or drug) is a chemical that directly modulates the excitatory amino acid (glutamate/aspartate) system in the body or brain. Examples include excitatory amino acid receptor agonis ...
receptors which are excitatory. The of glycine is 7930 mg/kg in rats (oral), and it usually causes death by hyperexcitability.
Uses
In the US, glycine is typically sold in two grades:United States Pharmacopeia
The ''United States Pharmacopeia'' (''USP'') is a pharmacopeia (compendium of drug information) for the United States published annually by the United States Pharmacopeial Convention (usually also called the USP), a nonprofit organization that ...
(“USP”), and technical grade. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine. If purity greater than the USP standard is needed, for example for intravenous injections, a more expensive pharmaceutical grade glycine can be used. Technical grade glycine, which may or may not meet USP grade standards, is sold at a lower price for use in industrial applications, e.g., as an agent in metal complexing and finishing.
Animal and human foods
 Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of
Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine
Saccharin (''aka'' saccharine, Sodium sacchari) is an artificial sweetener with effectively no nutritional value. It is about 550 times as sweet as sucrose but has a bitter or metallic aftertaste, especially at high concentrations. Saccharin i ...
. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate
Copper(II) glycinate (IUPAC suggested name: bis(glycinato)copper(II)) refers to the coordination complex of copper(II) with two equivalents of glycinate, with the formula u(glycinate)2(H2O)''x''where ''x'' = 1 (''monohydrate'') or 0 (''anhydrous ...
are used as supplements for animal feeds.
The U.S. "Food and Drug Administration no longer regards glycine and its salts as generally recognized as safe for use in human food".
Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicidesglyphosate
Glyphosate (IUPAC name: ''N''-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by inhibiting the plant enzyme 5-enolpyruvylshik ...
, iprodione
Iprodione is a hydantoin fungicide and nematicide.
Application
Iprodione is used on crops affected by Botrytis bunch rot, Brown rot, Sclerotinia and other fungal diseases in plants. It is currently applied in a variety of crops: fruit, vegetable ...
, glyphosine, imiprothrin
Imiprothrin is a synthetic pyrethroid insecticide. It is an ingredient in some commercial and consumer insecticide products for indoor use. It has low acute toxicity to humans through the inhalation and dermal routes, but to insects it acts as a ...
, and eglinazine. It is used as an intermediate of the medicine such as thiamphenicol
Thiamphenicol (also known as thiophenicol and dextrosulphenidol) is an antibiotic. It is the methyl- sulfonyl analogue of chloramphenicol and has a similar spectrum of activity, but is 2.5 to 5 times as potent. Like chloramphenicol, it is insolub ...
.
Laboratory research
Glycine is a significant component of some solutions used in theSDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a Discontinuous electrophoresis, discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular m ...
method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis. Glycine is also used to remove protein-labeling antibodies from Western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required. This process is known as stripping.
Presence in space
The presence of glycine outside the earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by theNASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the US federal government responsible for the civil List of government space agencies, space program ...
spacecraft '' Stardust'' from comet Wild 2
Comet 81P/Wild, also known as Wild 2 (pronounced "vilt two") ( ), is a comet named after Swiss astronomer Paul Wild, who discovered it on January 6, 1978, using a 40-cm Schmidt telescope at Zimmerwald, Switzerland.
For most of its 4.5 billion ...
and subsequently returned to earth. Glycine had previously been identified in the Murchison meteorite
The Murchison meteorite is a meteorite that fell in Australia in 1969 near Murchison, Victoria. It belongs to the carbonaceous chondrite class, a group of meteorites rich in organic compounds. Due to its mass (over ) and the fact that it was an ...
in 1970. The discovery of glycine in outer space bolstered the hypothesis of so called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the universe. In 2016, detection of glycine within Comet 67P/Churyumov–Gerasimenko
67P/Churyumov–Gerasimenko (abbreviated as 67P or 67P/C–G) is a Jupiter-family comet, originally from the Kuiper belt, with a current orbital period of 6.45 years, a rotation period of approximately 12.4 hours and a maximum velocity of . Chu ...
by the ''Rosetta'' spacecraft was announced.
The detection of glycine outside the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
in the interstellar medium has been debated. In 2008, the Max Planck Institute for Radio Astronomy
The Max Planck Institute for Radio Astronomy (MPIfRA) (German: ''Max-Planck-Institut für Radioastronomie'') is located in Bonn, Germany. It is one of 80 institutes in the Max Planck Society (German: Max-Planck-Gesellschaft).
History
By com ...
discovered the spectral lines of a glycine precursor (aminoacetonitrile
Aminoacetonitrile is the organic compound with the formula NCCH2NH2. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is ...
) in the Large Molecule Heimat
The Large Molecule Heimat is a dense gas cloud located in the molecular cloud Sagittarius B2. Many species of molecule, including aminoacetonitrile (a molecule related to glycine), ethyl formate, and butyronitrile
Butyronitrile or butanenitril ...
, a giant gas cloud near the galactic center in the constellation Sagittarius.
Evolution
Glycine is proposed to be defined by early genetic codes. For example, low complexity regions (in proteins), that may resemble the proto-peptides of the earlygenetic code
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
are highly enriched in glycine.
Presence in foods
See also
*Trimethylglycine
Trimethylglycine is an amino acid derivative that occurs in plants. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because, in the 19th century, it was discovered in sugar beets. It has a sweet and umami ...
* Amino acid neurotransmitter
An amino acid neurotransmitter is an amino acid which is able to transmit a nerve message across a synapse. Neurotransmitters (chemicals) are packaged into vesicles that cluster beneath the axon terminal membrane on the presynaptic side of a ...
References
Further reading
* *External links
Glycine MS Spectrum
* *
{{Authority control Flavor enhancers Glucogenic amino acids Inhibitory amino acids Proteinogenic amino acids Glycine receptor agonists NMDA receptor agonists E-number additives Pages including recorded pronunciations