Diagnosis of HIV/AIDS on:
[Wikipedia]
[Google]
[Amazon]
HIV tests are used to detect the presence of the
 Like the ELISA procedure, the
Like the ELISA procedure, the

Complete List of Donor Screening Assays for Infectious Agents and HIV Diagnostic Assays
– FDA * Fact sheets from the National Aids Trust ("NAT") in the UK: *
General information on HIV testing
â€
Types of HIV test
â€
Home testing
Bulk procurement of HIV test kits
instructions from the
human immunodeficiency virus
The human immunodeficiency viruses (HIV) are two species of ''Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immun ...
(HIV), the virus that causes acquired immunodeficiency syndrome
Human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS) is a spectrum of conditions caused by infection with the human immunodeficiency virus (HIV), a retrovirus. Following initial infection an individual ma ...
(AIDS), in serum
Serum may refer to:
* Serum (blood), plasma from which the clotting proteins have been removed
**Antiserum, blood serum with specific antibodies for passive immunity
* Serous fluid, any clear bodily fluid
*Truth serum, a drug that is likely to mak ...
, saliva
Saliva (commonly referred to as spit) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can ...
, or urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excreted from the body through the urethra.
Cellul ...
. Such tests may detect antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of ...
, antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune respon ...
s, or RNA.
AIDS diagnosis
AIDS is diagnosed separately from HIV.Terminology
Thewindow period
In medicine, the window period for a test designed to detect a specific disease (particularly infectious disease) is the time between first infection and when the test can reliably detect that infection. In antibody-based testing, the window peri ...
is the time from infection until a test can detect any change. The average window period with HIV-1 antibody tests is 25 days for subtype B. Antigen testing cuts the window period to approximately 16 days and nucleic acid testing
A nucleic acid test (NAT) is a technique used to detect a particular nucleic acid sequence and thus usually to detect and identify a particular species or subspecies of organism, often a virus or bacterium that acts as a pathogen in blood, tiss ...
(NAT) further reduces this period to 12 days.
Performance of medical tests is often described in terms of:
* Sensitivity: The percentage of the results that will be positive when HIV is present
* Specificity: The percentage of the results that will be negative when HIV is not present.
All diagnostic tests have limitations, and sometimes their use may produce erroneous or questionable results.
* False positive
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
: The test incorrectly indicates that HIV is present in a non-infected person.
* False negative
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
: The test incorrectly indicates that HIV is absent in an infected person.
Nonspecific reactions, hypergammaglobulinemia
Hypergammaglobulinemia is a medical condition with elevated levels of gamma globulin. It is a type of immunoproliferative disorder.
Types
Hypergammaglobulinemia is a condition that is characterized by the increased levels of a certain immunogl ...
, or the presence of antibodies directed to other infectious agents that may be antigenically similar to HIV can produce false positive results. Autoimmune diseases, such as systemic lupus erythematosus
Lupus, technically known as systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Commo ...
, have also rarely caused false positive results. Most false negative results are due to the window period.
Principles
Screening donor blood and cellular products
Tests selected to screen donor blood and tissue must provide a high degree of confidence that HIV will be detected if present (that is, a high sensitivity is required). A combination ofantibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of t ...
, antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune respon ...
and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main ...
tests are used by blood bank
A blood bank is a center where blood gathered as a result of blood donation is stored and preserved for later use in blood transfusion. The term "blood bank" typically refers to a department of a hospital usually within a Clinical Pathology laborat ...
s in Western countries. The World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level o ...
estimated that, , inadequate blood screening had resulted in 1 million new HIV infections worldwide.
In the US, the Food and Drug Administration requires that all donated blood be screened for several infectious diseases, including HIV-1 and HIV-2, using a combination of antibody testing (EIA Eia or EIA may refer to:
Medicine
* Enzyme immunoassay
* Equine infectious anemia
* Exercise-induced anaphylaxis
* Exercise-induced asthma
* External iliac artery
Transport
* Edmonton International Airport, in Alberta, Canada
* Erbil Internatio ...
) and more expeditious nucleic acid testing (NAT). These diagnostic tests are combined with careful donor selection. , the risk of transfusion-acquired HIV in the US was approximately one in 2.5 million for each transfusion.
Diagnosis of HIV infection
Tests used for the diagnosis of HIV infection in a particular person require a high degree of both sensitivity and specificity. In the United States, this is achieved using analgorithm
In mathematics and computer science, an algorithm () is a finite sequence of rigorous instructions, typically used to solve a class of specific problems or to perform a computation. Algorithms are used as specifications for performing ...
combining two tests for HIV antibodies. If antibodies are detected by an initial test based on the ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presen ...
method, then a second test using the western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
procedure determines the size of the antigens in the test kit binding to the antibodies. The combination of these two methods is highly accurate
Human rights
The UNAIDS/WHO policy statement on HIV Testing states that conditions under which people undergo HIV testing must be anchored in ahuman rights
Human rights are moral principles or normsJames Nickel, with assistance from Thomas Pogge, M.B.E. Smith, and Leif Wenar, 13 December 2013, Stanford Encyclopedia of PhilosophyHuman Rights Retrieved 14 August 2014 for certain standards of hu ...
approach that pays due respect to ethical principles. According to these principles, the conduct of HIV testing of individuals must be
* Confidential
Confidentiality involves a set of rules or a promise usually executed through confidentiality agreements that limits the access or places restrictions on certain types of information.
Legal confidentiality
By law, lawyers are often required ...
;
* Accompanied by counseling (for those who test positive);
* Conducted with the informed consent
Informed consent is a principle in medical ethics and medical law, that a patient must have sufficient information and understanding before making decisions about their medical care. Pertinent information may include risks and benefits of treat ...
of the person being tested.
Confidentiality
Considerable controversy exists over the ethical obligations of health care providers to inform the sexual partners of individuals infected with HIV that they are at risk of contracting the virus. Some legal jurisdictions permit such disclosure, while others do not. More state funded testing sites are now using confidential forms of testing. This allows for monitoring of infected individuals easily, compared to anonymous testing that has a number attached to the positive test results. Controversy exists over privacy issues. In developing countries, home-based HIV testing and counseling (HBHTC) is an emerging approach for addressing confidentiality issues. HBHTC allows individuals, couples, and families to learn their HIV status in the convenience and privacy of their home environment. Rapid HIV tests are most often used, so results are available for the client between 15 and 30 minutes. Furthermore, when an HIV-positive result is communicated, the HTC provider can offer appropriate linkages for prevention, care, and treatment.Anonymous testing
Testing that has only a number attached to the specimen that will be delivered for testing. Items that are confirmed positive will not have the HIV infected individual's name attached to the specimen. Sites that offer this service advertise this testing option.Routine testing recommendation
In the United States, one emerging standard of care is to screen all patients for HIV in all health care settings. In 2006, theCenters for Disease Control
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georgi ...
(CDC) announced an initiative for voluntary, routine testing of all Americans aged 13–64 during health care encounters. An estimated 25% of infected individuals were unaware of their status; if successful, this effort was expected to reduce new infections by 30% per year. The CDC recommends elimination of requirements for written consent or extensive pre-test counseling as barriers to widespread routine testing. In 2006, the National Association of Community Health Centers implemented a model for offering free, rapid HIV testing to all patients between the ages of 13 and 64 during routine primary medical and dental care visits. The program increased testing rates, with 66% of the 17,237 patients involved in the study agreeing to testing (56% were tested for the first time). In September 2010, New York became the first state to require that hospitals and primary care providers offer an HIV test to all patients between the ages of 13 and 64 years. An evaluation of the law's impact found that it increased testing significantly throughout the state.
Antibody tests
HIV antibody tests are specifically designed for routine diagnostic testing of adults; these tests are inexpensive and extremely accurate.Window period
Antibody tests may givefalse negative
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
(no antibodies were detected despite the presence of HIV) results during the ''window period
In medicine, the window period for a test designed to detect a specific disease (particularly infectious disease) is the time between first infection and when the test can reliably detect that infection. In antibody-based testing, the window peri ...
'', an interval of three weeks to six months between the time of HIV infection and the production of measurable antibodies to HIV seroconversion
In immunology, seroconversion is the development of specific antibodies in the blood serum as a result of infection or immunization, including vaccination. During infection or immunization, antigens enter the blood, and the immune system begins t ...
. Most people develop detectable antibodies approximately 30 days after infection, although some seroconvert later. The vast majority of people (97%) have detectable antibodies by three months after HIV infection; a six-month window is extremely rare with modern antibody testing. During the window period, an infected person can transmit HIV to others although their HIV infection may not be detectable with an antibody test. Antiretroviral therapy
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multi ...
during the window period can delay the formation of antibodies and extend the window period beyond 12 months. This was not the case with patients that underwent treatment with post-exposure prophylaxis
Post-exposure prophylaxis, also known as post-exposure prevention (PEP), is any preventive medical treatment started after exposure to a pathogen in order to prevent the infection from occurring.
COVID-19
In 2021, the FDA has approved bamlanivi ...
(PEP). Those patients must take ELISA tests at various intervals after the usual 28-day course of treatment, sometimes extending outside of the conservative window period of 6 months. Antibody tests may also yield false negative results in patients with X-linked agammaglobulinemia
X-linked agammaglobulinemia (XLA) is a rare genetic disorder discovered in 1952 that affects the body's ability to fight infection. As the form of agammaglobulinemia that is X-linked, it is much more common in males. In people with XLA, the whit ...
; other diagnostic tests should be used in such patients.
Three instances of delayed HIV seroconversion occurring in health-care workers have been reported; in these instances, the health-care workers tested negative for HIV antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of ...
greater than 6 months postexposure but were seropositive within 12 months after the exposure. DNA sequencing confirmed the source of infection in one instance. Two of the delayed seroconversions were associated with simultaneous exposure to hepatitis C
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection people often have mild or no symptoms. Occasionally a fever, dark urine, ...
virus (HCV). In one case, co-infection was associated with a rapidly fatal HCV disease course; however, it is not known whether HCV directly influences the risk for or course of HIV infection or is a marker for other exposure-related factors.
ELISA
The ''enzyme-linked immunosorbent assay'' (ELISA), or ''enzyme immunoassay'' (EIA), was the first screening test commonly employed for HIV. It has a high sensitivity. In anELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presen ...
test, a person's serum
Serum may refer to:
* Serum (blood), plasma from which the clotting proteins have been removed
**Antiserum, blood serum with specific antibodies for passive immunity
* Serous fluid, any clear bodily fluid
*Truth serum, a drug that is likely to mak ...
is diluted 400-fold and applied to a plate to which HIV antigens have been attached. If antibodies to HIV are present in the serum, they may bind to these HIV antigens. The plate is then washed to remove all other components of the serum. A specially prepared " secondary antibody" – an antibody that binds to human antibodies – is then applied to the plate, followed by another wash. This secondary antibody is chemically linked in advance to an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
. Thus the plate will contain enzyme in proportion to the amount of secondary antibody bound to the plate. A substrate for the enzyme is applied, and catalysis by the enzyme leads to a change in color or fluorescence. ELISA results are reported as a number; the most controversial aspect of this test is determining the "cut-off" point between a positive and negative result.
ELISA dongle
Researchers fromColumbia University
Columbia University (also known as Columbia, and officially as Columbia University in the City of New York) is a private research university in New York City. Established in 1754 as King's College on the grounds of Trinity Church in Manhatt ...
have produced an ELISA test dongle
A dongle is a small piece of computer hardware that connects to a port on another device to provide it with additional functionality, or enable a pass-through to such a device that adds functionality.
In computing, the term was initially synonym ...
capable of testing for HIV and syphilis
Syphilis () is a sexually transmitted infection caused by the bacterium '' Treponema pallidum'' subspecies ''pallidum''. The signs and symptoms of syphilis vary depending in which of the four stages it presents (primary, secondary, latent, a ...
. It is compatible to any smartphone
A smartphone is a portable computer device that combines mobile telephone and computing functions into one unit. They are distinguished from feature phones by their stronger hardware capabilities and extensive mobile operating systems, whi ...
or computer without additional support or battery power, and takes some fifteen minutes to analyse a drop of blood. The units cost approximately $34 each to manufacture.
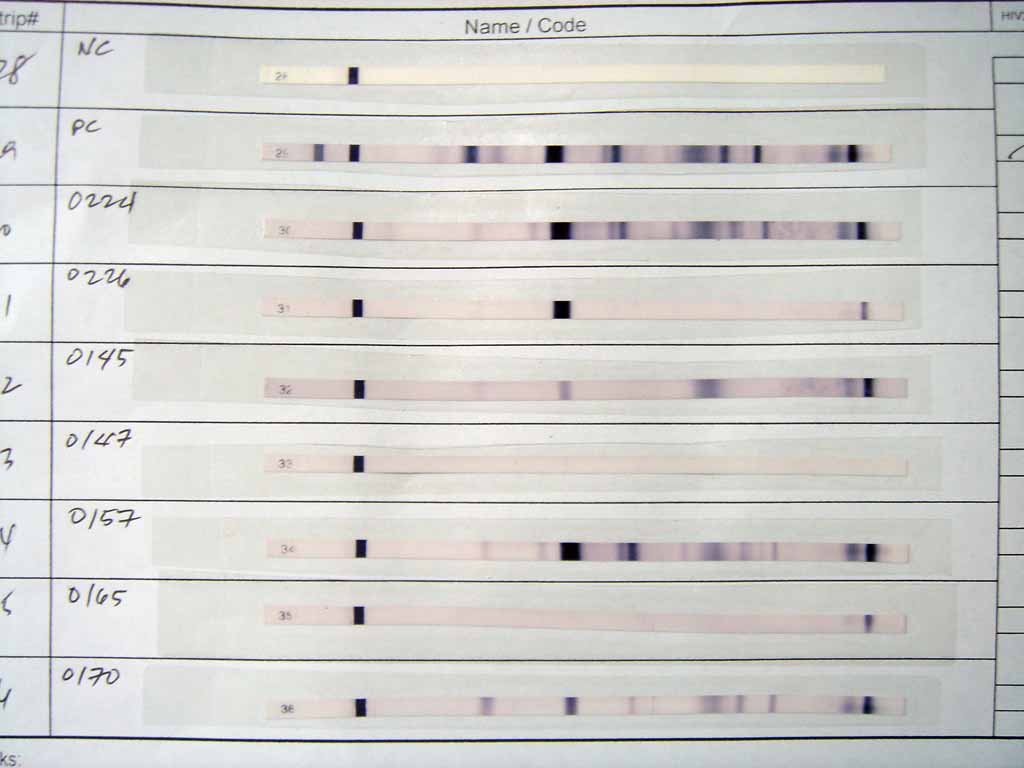
Western blot
 Like the ELISA procedure, the
Like the ELISA procedure, the western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
is an antibody detection test. However, unlike the ELISA method, the viral proteins are separated first and immobilized. In subsequent steps, the binding of serum antibodies to specific HIV proteins is visualized.
Specifically, cells that may be HIV-infected are opened and the protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s within are placed into a slab of gel, to which an electric current is applied. Different proteins will move with different speeds in this field, depending on their size, while their electrical charge is leveled by a surfactant called sodium lauryl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium s ...
. Some commercially prepared Western blot test kits contain the HIV proteins already on a cellulose acetate strip. Once the proteins are well-separated, they are transferred to a membrane and the procedure continues similar to an ELISA: the person's diluted serum is applied to the membrane and antibodies in the serum may attach to some of the HIV proteins. Antibodies that do not attach are washed away, and enzyme-linked antibodies with the capability to attach to the person's antibodies determine to which HIV proteins the person has antibodies.
There are no universal criteria for interpreting the western blot test: The number of viral bands that must be present may vary. If no viral bands are detected, the result is negative. If at least one viral band for each of the GAG, POL, and ENV gene-product groups are present, the result is positive. The three-gene-product approach to western blot interpretation has not been adopted for public health or clinical practice. Tests in which less than the required number of viral bands are detected are reported as indeterminate: a person who has an indeterminate result should be retested, as later tests may be more conclusive. Almost all HIV-infected persons with indeterminate western blot results will develop a positive result when tested in one month; persistently indeterminate results over a period of six months suggests the results are not due to HIV infection. In a generally healthy low-risk population, indeterminate results on western blot occur on the order of 1 in 5,000 patients. However, for those individuals who have had high-risk exposures to individuals where HIV-2 is most prevalent, Western Africa, an inconclusive western blot test may prove infection with HIV-2.
The HIV proteins used in western blotting can be produced by recombinant DNA
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be f ...
in a technique called ''recombinant immunoblot assay'' (RIBA).
Rapid or point-of-care tests

Rapid
Rapids are sections of a river where the river bed has a relatively steep gradient, causing an increase in water velocity and turbulence.
Rapids are hydrological features between a ''run'' (a smoothly flowing part of a stream) and a ''cascade'' ...
antibody tests are qualitative immunoassays intended for use in point-of-care testing to aid in the diagnosis of HIV infection. These tests should be used in conjunction with the clinical status, history, and risk factors of the person being tested. The positive predictive value
The positive and negative predictive values (PPV and NPV respectively) are the proportions of positive and negative results in statistics and diagnostic tests that are true positive and true negative results, respectively. The PPV and NPV des ...
of Rapid Antibody Tests in low-risk populations has not been evaluated. These tests should be used in appropriate multi-test algorithm
In mathematics and computer science, an algorithm () is a finite sequence of rigorous instructions, typically used to solve a class of specific problems or to perform a computation. Algorithms are used as specifications for performing ...
s designed for statistical validation of rapid HIV test results.
If no antibodies to HIV are detected, this does not mean the person has not been infected with HIV. It may take several months after HIV infection for the antibody response to reach detectable levels, during which time rapid testing for antibodies to HIV will not be indicative of true infection status. For most people, HIV antibodies reach a detectable level after two to six weeks.
Although these tests have high specificity, false positives do occur. Any positive test result should be confirmed by a lab using the western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
.
Interpreting antibody tests
ELISA testing alone cannot be used to diagnose HIV, even if the test suggests a high probability that antibody to HIV-1 is present. In the United States, such ELISA results are not reported as "positive" unless confirmed by a western blot. The ELISA antibody tests were developed to provide a high level of confidence that donated blood was ''not'' infected with HIV. It is therefore not possible to conclude that blood rejected for transfusion because of a ''positive'' ELISA antibody test is in fact infected with HIV. Sometimes, retesting the donor in several months will produce a ''negative'' ELISA antibody test. This is why a confirmatory western blot is always used before reporting a "positive" HIV test result. Rare false positive results due to factors unrelated to HIV exposure are found more often with the ELISA test than with the western blot. False positives may be associated with medical conditions such as recent acute illnesses and allergies. A rash of false positive tests in the fall of 1991 was initially blamed on the influenza vaccines used during that flu season, but further investigation traced the cross-reactivity to several relatively non-specific test kits. A false positive result does not indicate a condition of significant risk to health. When the ELISA test is combined with Western Blot, the rate of false positives is extremely low, and diagnostic accuracy is very high (see below). :HIV antibody tests are highly sensitive, meaning they react preferentially with HIV antibodies, but not all positive or inconclusive HIV ELISA tests mean the person is infected by HIV. Risk history, and clinical judgement should be included in the assessment, and a confirmation test (western blot) should be administered. An individual with an inconclusive test should be re-tested at a later date.Accuracy of HIV testing
Modern HIV testing is highly accurate. The evidence regarding the risks and benefits of HIV screening was reviewed in July 2005 by the U.S. Preventive Services Task Force. The authors concluded that: The specificity rate given here for the inexpensive enzyme immunoassay screening tests indicates that, in 1,000 HIV test results of healthy individuals, about 15 of these results will be afalse positive
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
. Confirming the test result (i.e., by repeating the test, if this option is available) could reduce the ultimate likelihood of a false positive to about 1 result in 250,000 tests given. The sensitivity rating, likewise, indicates that, in 1,000 test results of HIV infected people, 3 will actually be a false negative
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
result. However, based upon the HIV prevalence rates at most testing centers within the United States, the negative predictive value
The positive and negative predictive values (PPV and NPV respectively) are the proportions of positive and negative results in statistics and diagnostic tests that are true positive and true negative results, respectively. The PPV and NPV des ...
of these tests is extremely high, meaning that a negative test result will be correct more than 9,997 times in 10,000 (99.97% of the time). The very high negative predictive value of these tests is why the CDC recommends that a negative test result be considered conclusive evidence that an individual does not have HIV.
Of course, the actual numbers vary depending on the testing population. This is because interpreting of the results of any medical test (assuming no test is 100% accurate) depends upon the initial degree of belief, or the prior probability
In Bayesian statistical inference, a prior probability distribution, often simply called the prior, of an uncertain quantity is the probability distribution that would express one's beliefs about this quantity before some evidence is taken into ...
that an individual has, or does not have a disease. Generally the prior probability is estimated using the prevalence of a disease within a population or at a given testing location. The positive predictive value
The positive and negative predictive values (PPV and NPV respectively) are the proportions of positive and negative results in statistics and diagnostic tests that are true positive and true negative results, respectively. The PPV and NPV des ...
and negative predictive value of all tests, including HIV tests, take into account the prior probability of having a disease along with the accuracy of the testing method to determine a new degree of belief that an individual has or does not have a disease (also known as the posterior probability
The posterior probability is a type of conditional probability that results from updating the prior probability with information summarized by the likelihood via an application of Bayes' rule. From an epistemological perspective, the posterior p ...
). The chance that a positive test accurately indicates an HIV infection increases as the prevalence or rate of HIV infection increases in the population. Conversely, the negative predictive value will decrease as the HIV prevalence rises. Thus a positive test in a high-risk population, such as people who frequently engage in unprotected anal intercourse with unknown partners, is more likely to correctly represent HIV infection than a positive test in a very low-risk population, such as unpaid blood donors.
Many studies have confirmed the accuracy of current methods of HIV testing in the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
, reporting false-positive rates of 0.0004 to 0.0007 and false-negative rates of 0.003 in the general population.Update: serologic testing for HIV-1 antibody – United States, 1988 and 1989. ''MMWR Morb Mortal Wkly Rep'' 1990; 39:380.
Antigen tests
The p24 antigen test detects the presence of the p24 protein of HIV (also known as CA), the capsid protein of the virus.Monoclonal antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Monoclonal antibodies ...
specific to the p24 protein are mixed with the person's blood. Any p24 protein in the person's blood will stick to the monoclonal antibody and an enzyme-linked antibody to the monoclonal antibodies to p24 causes a color change if p24 was present in the sample.
In blood donation screening, this test is no longer used routinely in the US or the EU since the objective was to reduce the risk of false negatives in the window period. Nucleic acid testing (NAT) is more effective for this purpose, and p24 antigen testing is no longer indicated if a NAT test is performed.
In general diagnostics, p24 antigen tests are used for early detection of HIV, as p24 antigen rises soon after infection relative to antibodies, and the test is often used in combination with an antibody test to effectively cover a longer portion of the window period. It is less useful as a standalone test, as it has low sensitivity and only works during the early time period after infection. The presence of p24 antigen diminishes as the body increases production of antibodies to the p24 protein, making p24 more difficult to detect later.
Antigen/antibody combination tests
A combination, or 4th generation assay, is designed to detect both the p24 antigen and HIV antibodies in a single test. Combination tests can detect HIV as early as 2–6 weeks after infection, and are recommended in laboratory testing.Nucleic acid-based tests (NAT)
Nucleic-acid-based tests amplify and detect one or more of several target sequences located in specific HIV genes, such as HIV-I GAG, HIV-II GAG, HIV-env, or the HIV-pol. Since these tests are relatively expensive, the blood is screened by first pooling some 8–24 samples and testing these together; if the pool tests positive, each sample is retested individually. Although this results in a dramatic decrease in cost, the dilution of the virus in the pooled samples decreases the effective sensitivity of the test, lengthening the window period by four days (assuming a 20-fold dilution, ~20hr virus doubling time, detection limit 50 copies/ml, making limit of detection 1,000 copies/ml). Since 2001, donated blood in the United States has been screened with nucleic-acid-based tests, shortening the window period between infection and detectability of disease to a median of 17 days (95% CI, 13–28 Days, assumes pooling of samples). A different version of this test is intended for use in conjunction with clinical presentation and other laboratory markers of disease progress for the management of -infected patients. In the RT-PCR test, viral RNA is extracted from the patient's plasma and is treated withreverse transcriptase
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genom ...
(RT) to convert the viral RNA into cDNA
In genetics, complementary DNA (cDNA) is DNA synthesized from a single-stranded RNA (e.g., messenger RNA (mRNA) or microRNA (miRNA)) template in a reaction catalyzed by the enzyme reverse transcriptase. cDNA is often used to express a sp ...
. The polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) ...
(PCR) process is then applied, using two primers unique to the virus's genome. After PCR amplification is complete, the resulting DNA products are hybridized to specific oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small bits of nucleic acids ...
s bound to the vessel wall, and are then made visible with a probe bound to an enzyme. The amount of virus in the sample can be quantified with sufficient accuracy to detect threefold changes.
In the Quantiplex bDNA or branched DNA test, plasma is placed in a centrifuge
A centrifuge is a device that uses centrifugal force to separate various components of a fluid. This is achieved by spinning the fluid at high speed within a container, thereby separating fluids of different densities (e.g. cream from milk) or ...
to concentrate the virus, which is then opened to release its RNA. Special oligonucleotides that bind to viral RNA and to certain oligonucleotides bound to the wall of the vessel are added. In this way, viral RNA is fastened to the wall. Then new oligonucleotides that bind at several locations to this RNA are added, and other oligonucleotides that bind at several locations to those oligonucleotides. This is done to amplify the signal. Finally, oligonucleotides that bind to the last set of oligonucleotides and that are bound to an enzyme are added; the enzyme action causes a color reaction, which allows quantification of the viral RNA in the original sample. Monitoring the effects of antiretroviral therapy by serial measurements of plasma HIV-1 RNA with this test has been validated for patients with viral loads greater than 25,000 copies per milliliter.
Screening
The South African government announced a plan to start screening for HIV in secondary schools by March 2011. This plan was cancelled due to concerns it would invade pupils' privacy, schools typically don't have the facilities to securely store such information, and schools generally do not have the capacity to provide counseling for HIV-positive pupils. In South Africa, anyone over the age of 12 may request an HIV test without parental knowledge or consent. Some 80,000 pupils in three provinces were tested under this programme before it ended.Other tests used in HIV treatment
The CD4 T-cell count is not an HIV test, but rather a procedure where the number of CD4 T-cells in the blood is determined. A CD4 count does not check for the presence of HIV. It is used to monitor immune system function in HIV-positive people. Declining CD4 T-cell counts are considered to be a marker of progression of HIV infection. A normal CD4 count can range from 500 cells/mm3 to 1000 cells/mm3. In HIV-positive people, AIDS is officially diagnosed when the count drops below 200 cells/ÎĽL or when certainopportunistic infection
An opportunistic infection is an infection caused by pathogens (bacteria, fungi, parasites or viruses) that take advantage of an opportunity not normally available. These opportunities can stem from a variety of sources, such as a weakened immun ...
s occur. This use of a CD4 count as an AIDS criterion was introduced in 1992; the value of 200 was chosen because it corresponded with a greatly increased likelihood of opportunistic infection. Lower CD4 counts in people with AIDS are indicators that prophylaxis against certain types of opportunistic infections should be instituted.
Low CD4 T-cell counts are associated with a variety of conditions, including many viral infections, bacterial infections, parasitic infections, primary immunodeficiency, coccidioidomycosis, burns, trauma, intravenous injections of foreign proteins, malnutrition, over-exercising, pregnancy, normal daily variation, psychological stress, and social isolation.
This test is also used occasionally to estimate immune system function for people whose CD4 T cells are impaired for reasons other than HIV infection, which include several blood diseases, several genetic disorders, and the side effects of many chemotherapy drugs.
In general, the lower the number of T cells the lower the immune system's function will be. Normal CD4 counts are between 500 and 1500 CD4+ T cells/microliter, and the counts may fluctuate in healthy people, depending on recent infection status, nutrition, exercise, and other factors. Women tend to have somewhat lower counts than men.
Criticisms
Oral tests
As a result of an increase in false positive rates with rapid oral HIV testing in 2005, New York City's Department of Health and Mental Hygiene added the option of testing finger-stick whole blood after any reactive result, before using a western blot test to confirm the positive result. Following a further increase of false positives in NYC DOHMH STD Clinics during the end of 2007 and beginning of 2008, their clinics opted to forgo further oral screenings, and instead reinsituted testing using finger-stick whole blood. Despite the increase in false positives in NYC DOHMH, the CDC still continues to support the use of noninvasive oral fluid specimens due to their popularity in health clinics and convenience of use. The director of the HIV control program for public health at Seattle King county, reported OraQuick failed to spot at least 8 percent of 133 people found to be infected with a comparable diagnostic test. Strategies implemented to determine quality control and false positive rates were implemented. It is to be understood that any reactive OraQuick test result is a preliminary positive result and will always require a confirmatory test, regardless of the mean of testing (venipuncture whole blood, fingerstick whole blood or oral mucosal transudate fluid). Several other testing sites who did not experience a spike in false positive rates continue to use OraSure's OraQuick HIV Anti-body Testing.AIDS denialism
HIV tests have been criticized byAIDS denialists
HIV/AIDS denialism is the belief, despite conclusive evidence to the contrary, that the human immunodeficiency virus (HIV) does not cause acquired immune deficiency syndrome (AIDS). Some of its proponents reject the existence of HIV, while oth ...
(a fringe group whose members believe that HIV either does not exist or is harmless). The accuracy of serologic testing has been verified by isolation and culture of HIV and by detection of HIV RNA by PCR, which are widely accepted " gold standards" in microbiology
Microbiology () is the scientific study of microorganisms, those being unicellular (single cell), multicellular (cell colony), or acellular (lacking cells). Microbiology encompasses numerous sub-disciplines including virology, bacteriology, ...
. While AIDS denialists focus on individual components of HIV testing, the combination of ELISA and western blot used for the diagnosis of HIV is remarkably accurate, with very low false-positive and -negative rates as described above. The views of AIDS denialists are based on highly selective analysis of mostly outdated scientific papers; there is broad scientific consensus
Scientific consensus is the generally held judgment, position, and opinion of the majority or the supermajority of scientists in a particular field of study at any particular time.
Consensus is achieved through scholarly communication at confe ...
that HIV is the cause of AIDS.
Fraudulent testing
There have been a number of cases of fraudulent tests being sold via mail order or the Internet to the general public. In 1997, a California man was indicted on mail fraud and wire charges for selling supposed home test kits. In 2004, the US Federal Trade Commission asked Federal Express and US Customs to confiscate shipments of the Discreet home HIV test kits, produced by Gregory Stephen Wong of Vancouver, Canada. In February 2005, the US FDA issued a warning against using the rapid HIV test kits and other home use kits marketed by Globus Media of Montreal, Canada.References
External links
Complete List of Donor Screening Assays for Infectious Agents and HIV Diagnostic Assays
– FDA * Fact sheets from the National Aids Trust ("NAT") in the UK: *
General information on HIV testing
â€
Types of HIV test
â€
Home testing
Bulk procurement of HIV test kits
instructions from the
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level o ...
{{DEFAULTSORT:Hiv Test
Infectious disease blood tests
HIV/AIDS