Demethylation on:
[Wikipedia]
[Google]
[Amazon]
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms.
The counterpart of demethylation is methylation.
 Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to reflux in a solution of
Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to reflux in a solution of 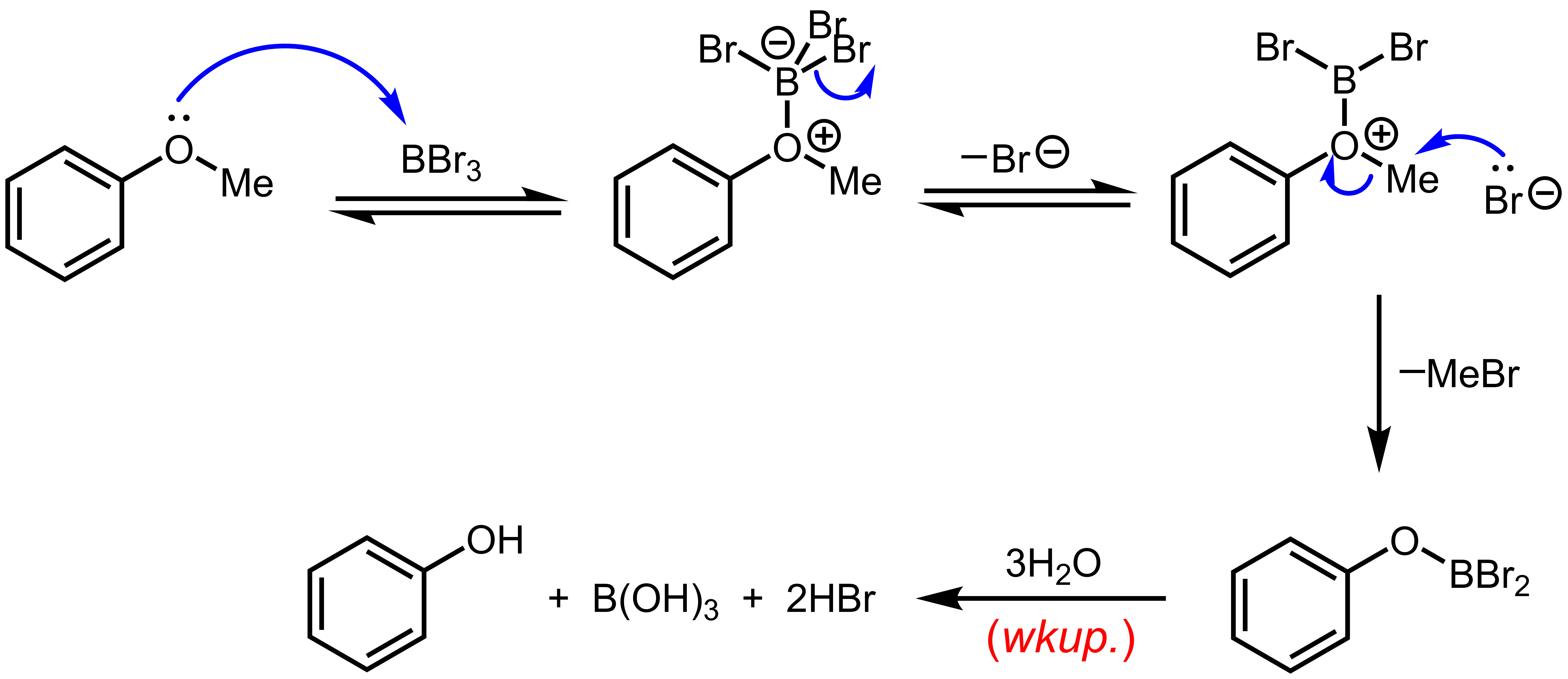 Methyl esters also are susceptible to demethylation, which is usually achieved by saponification. Highly specialized demethylations are abundant, such as the
Methyl esters also are susceptible to demethylation, which is usually achieved by saponification. Highly specialized demethylations are abundant, such as the  A mixture of
A mixture of
In biochemistry
Inbiochemical
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology an ...
systems, the process of demethylation is catalyzed by demethylases. These enzymes oxidize
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
N-methyl groups, which occur in histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn a ...
s and some forms of DNA:
:R2N-CH3 + O → R2N-H + CH2O
One such oxidative enzyme family is the cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various co ...
. Alpha-ketoglutarate-dependent hydroxylases are active for demethylation of DNA, operating by a similar pathway. These reactions exploit the weak C-H bond adjacent to amines.
In particular, 5-methylcytosines in DNA can be demethylated by TET enzymes as illustrated in the figure. TET enzymes are dioxygenase
Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine ...
s in the family of alpha-ketoglutarate-dependent hydroxylases. A TET enzyme is an alpha-ketoglutarate (α-KG) dependent dioxygenase that catalyses an oxidation reaction by incorporating a single oxygen atom from molecular oxygen (O2) into its substrate, 5-methylcytosine in DNA (5mC), to produce the product 5-hydroxymethylcytosine in DNA. This conversion is coupled with the oxidation of the co-substrate α-KG to succinate and carbon dioxide (see figure).
The first step involves the binding of α-KG and 5-methylcytosine to the TET enzyme active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate ( binding site) ...
. The TET enzymes each harbor a core catalytic domain with a double-stranded β-helix fold that contains the crucial metal-binding residues found in the family of Fe(II)/α-KG- dependent oxygenases
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14.
Discoverers
Oxygenases were disco ...
. α-KG coordinates as a bidentate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
(connected at two points) to Fe(II) (see figure), while the 5mC is held by a noncovalent force in close proximity. The TET active site contains a highly conserved triad motif, in which the catalytically-essential Fe(II) is held by two histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the ...
residues and one aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
residue (see figure). The triad binds to one face of the Fe center, leaving three labile sites available for binding α-KG and O2 (see figure). TET then acts to convert 5-methylcytosine to 5-hydroxymethylcytosine, while α-ketoglutarate is converted to succinate and CO2.
Demethylation of some sterol
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the go ...
s are steps in the biosynthesis of testosterone and cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell memb ...
. Methyl groups are lost as formate.
During embryogenesis
An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male spe ...
in the mouse, about 20 million 5-methylcytosines are demethylated in a six-hour period just after fertilization of an egg by a sperm to form a zygote.
In organic chemistry
Demethylation often refers to cleavage of ethers, especially aryl ethers, although there are some exceptions, such as ''N''-demethylation of amines (e.g. imipramine to desipramine).Cleaving methyl ethers
Aryl methyl ethers are pervasive inlignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity a ...
and many derived compounds. The demethylation of these materials has been the subject of much effort. The reaction typically requires harsh conditions or harsh reagents. For example, the methyl ether in vanillin
Vanillin is an organic compound with the molecular formula . It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now u ...
can be removed by heating near with strong base. Stronger nucleophiles such as diorganophosphides (LiPPh2) also cleave aryl ethers under milder conditions. Other strong nucleophiles that have been employed include thiolate salts like EtSNa.
Acidic conditions can also be used. Historically, aryl methyl ethers, including natural products such as codeine
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, ''Papaver somniferum''. It is typically ...
(''O''-methylmorphine), have been demethylated by heating the substance in molten pyridine hydrochloride (melting point ) at , sometimes with excess hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chlorid ...
, in a process known as the ''Zeisel–Prey ether cleavage''. Quantitative analysis for aromatic methyl ethers can be performed by argentometric determination of the ''N''-methylpyridinium chloride formed. The mechanism of this reaction starts with proton transfer from pyridinium ion to the aryl methyl ether, a highly unfavorable step (''K'' < 10–11) that accounts for the harsh conditions required, given the much weaker acidity of pyridinium ( p''K''a = 5.2) compared to the protonated aryl methyl ether (an arylmethyloxonium ion, p''K''a = –6.7 for aryl = Ph). This is followed by SN2 attack of the arylmethyloxonium ion at the methyl group by either pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
or chloride ion (depending on the substrate) to give the free phenol and, ultimately, ''N''-methylpyridinium chloride, either directly or by subsequent methyl transfer from methyl chloride to pyridine. Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to reflux in a solution of
Another classical (but, again, harsh) method for the removal of the methyl group of an aryl methyl ether is to heat the ether to reflux in a solution of hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room tem ...
or hydrogen iodide in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
(boiling point ) or concentrated hydrobromic or hydroiodic acid. The cleavage of ethers by hydrobromic or hydroiodic acid proceeds by a very similar mechanism, in which the highly acidic HBr or HI serves to protonate the ether, followed by displacement by bromide or iodide, both of which are excellent nucleophiles. A slightly milder set of conditions uses cyclohexyl iodide (CyI, 10.0 equiv) in ''N'',''N''-dimethylformamide to generate a small amount of hydrogen iodide '' in situ''. Boron tribromide, which can be used at room temperature or below, is a more specialized reagent for the demethylation of aryl methyl ethers. The mechanism of ether dealkylation proceeds via the initial reversible formation of a Lewis acid-base adduct between the strongly Lewis acidic BBr3 and the Lewis basic ether. This Lewis adduct can reversibly dissociate to give a dibromoboryl oxonium cation and Br–. Rupture of the ether linkage occurs through the subsequent nucleophilic attack on the oxonium species by Br– to yield an aryloxydibromoborane and methyl bromide. Upon completion of the reaction, the phenol is liberated along with boric acid (H3BO3) and hydrobromic acid (aq. HBr) upon hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
of the dibromoborane derivative during aqueous workup.
 Methyl esters also are susceptible to demethylation, which is usually achieved by saponification. Highly specialized demethylations are abundant, such as the
Methyl esters also are susceptible to demethylation, which is usually achieved by saponification. Highly specialized demethylations are abundant, such as the Krapcho decarboxylation
The Krapcho decarboxylation is the chemical reaction of esters with halide anions. The ester must contain an electron-withdrawing group in the beta position, such as β-ketoesters, malonic esters, α-cyanoesters, or α-sulfonylesters. It works be ...
:
:anethole
Anethole (also known as anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils. It is in the class of phe ...
, KOH, and alcohol was heated in an autoclave. Although the product of this reaction was the expected anol, a highly reactive dimerization product in the mother liquors called dianol was also discovered by Charles Dodds
Sir Edward Charles Dodds, 1st Baronet (13 October 1899 – 16 December 1973) was a British biochemist.
Personal life
He was born in Liverpool in 1899, the only child of Ralph Edward Dodds, a shoe retailer, and Jane (née Pack) Dodds. The family ...
.
''N''-demethylation
''N''-demethylation of 3° amines is by the von Braun reaction, which uses BrCN as the reagent to give the corresponding '' nor-'' derivatives. A modern variation of the von Braun reaction was developed, where BrCN was superseded by ethyl chloroformate. The preparation of Paxil from arecoline is an application of this reaction, as well as the synthesis of GSK-372,475, for example.See also
* Methylation, the addition of a methyl group to a substrateReferences
{{Reflist, 30em Gene expression Organic reactions