Cyclobutadiene on:
[Wikipedia]
[Google]
[Amazon]
Cyclobutadiene is an
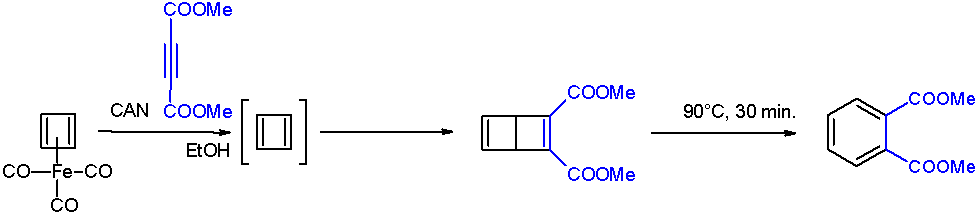 The Dewar benzene converts to dimethyl phthalate on heating at 90 °C.
One cyclobutadiene derivative is also accessible through a +2 ycloaddition of a di-
The Dewar benzene converts to dimethyl phthalate on heating at 90 °C.
One cyclobutadiene derivative is also accessible through a +2 ycloaddition of a di-
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coeffic ...
process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
Structure and reactivity
The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n'' annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence isomers. This distortion indicates that the pi electrons are localized, in agreement with Hückel's rule which predicts that a π-system of 4 electrons is notaromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
.
In principle, another situation is possible. Namely, cyclobutadiene could assume an undistorted square geometry, ''if it'' ''adopts a triplet spin state''. While a theoretical possibility, the triplet form of the parent cyclobutadiene and its substituted derivatives remained elusive for decades. However, in 2017, the square triplet excited state of 1,2,3,4-tetrakis(trimethylsilyl)-1,3-cyclobutadiene was observed spectroscopically, and a singlet-triplet gap of ''E''ST = 13.9 kcal/mol (or 0.6 eV per molecule) was measured for this compound.
Synthesis
Several cyclobutadiene derivatives have been isolated with steric bulky substituents. Orange tetrakis ( ''tert''-butyl)cyclobutadiene arises bythermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is re ...
of its isomer tetra-''tert''-butyltetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have b ...
. Although the cyclobutadiene derivative is stable (with respect to dimerization), it decomposes upon contact with .
Trapping
Samples of cyclobutadiene are unstable since the compounddimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
izes at temperatures above 35 K by a Diels-Alder reaction. By suppressing bimolecular decomposition pathways, cyclobutadiene is well-behaved. Thus it has been generated in a hemicarceplex. The inclusion compound
In host–guest chemistry In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full cova ...
is generated by photodecarboxylation of bicyclopyran-2-one. When released from the host–guest complex, cyclobutadiene dimerizes and then converts to cyclooctatetraene.
After numerous attempts, cyclobutadiene was first generated by oxidative degradation of cyclobutadieneiron tricarbonyl with ammonium cerium(IV) nitrate
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula . This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Preparation, properties, ...
. When liberated from the iron complex, cyclobutadiene reacts with electron-deficient alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s to form a Dewar benzene
Dewar benzene (also spelled ''dewarbenzene'') or bicyclo .2.0exa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures ...
:
: The Dewar benzene converts to dimethyl phthalate on heating at 90 °C.
One cyclobutadiene derivative is also accessible through a +2 ycloaddition of a di-
The Dewar benzene converts to dimethyl phthalate on heating at 90 °C.
One cyclobutadiene derivative is also accessible through a +2 ycloaddition of a di-alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
. In this particular reaction the trapping reagent is ''2,3,4,5-tetraphenylcyclopenta-2,4-dienone'' and one of the final products (after expulsion of carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
) is a cyclooctatetraene:
:
See also
*Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two v ...
*Cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared ...
References
{{Annulenes Annulenes Antiaromatic compounds Four-membered rings