Crosslink on:
[Wikipedia]
[Google]
[Amazon]
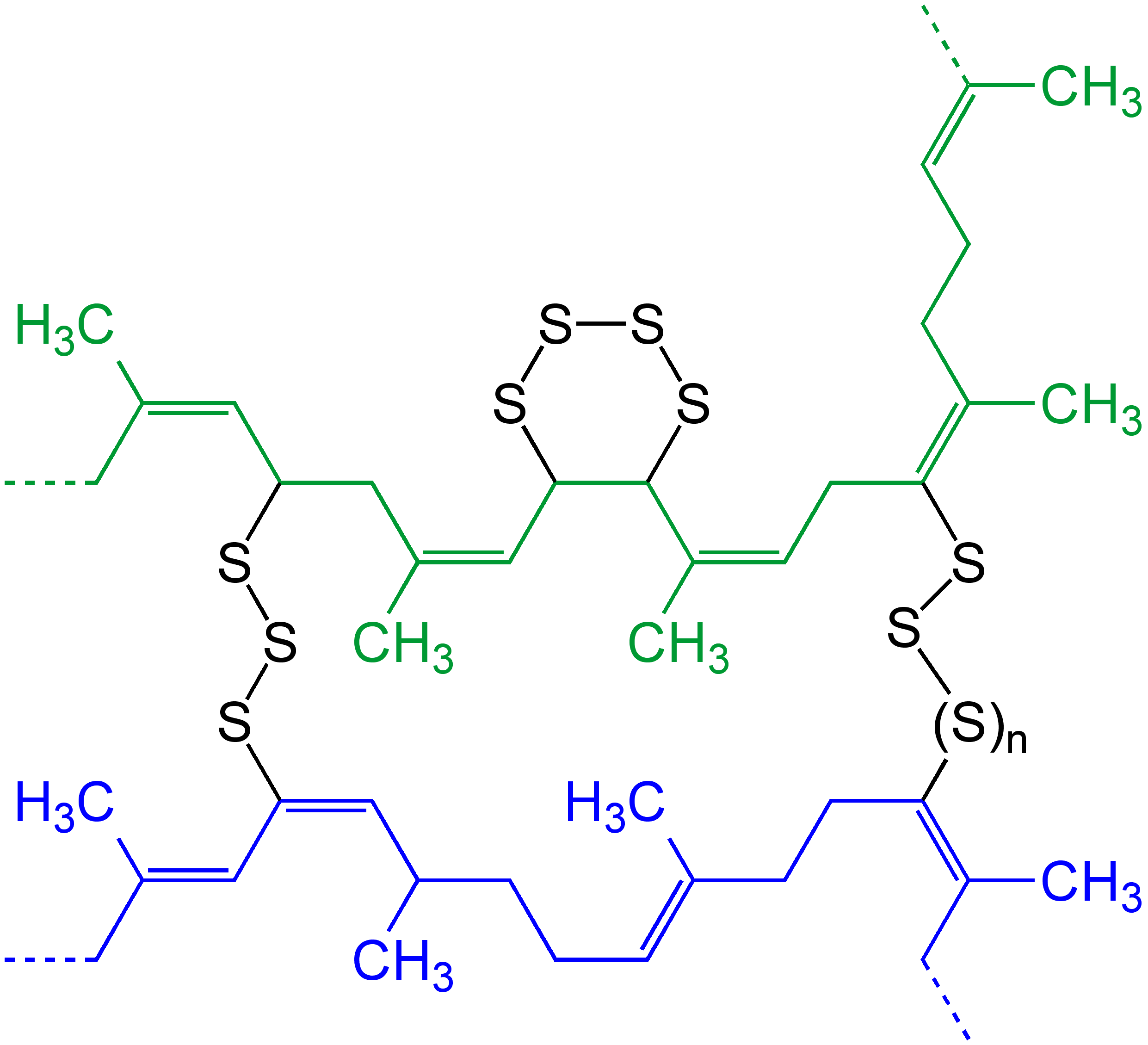 In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one

 Note: A rubber which cannot be reformed by heat or chemical treatment is called a thermoset elastomer. On the other hand, a thermoplastic elastomer can be molded and recycled by heat.
Note: A rubber which cannot be reformed by heat or chemical treatment is called a thermoset elastomer. On the other hand, a thermoplastic elastomer can be molded and recycled by heat.
Application note on how to measure degree of crosslinking in plastics
Ageing processes Polymer chemistry Protein–protein interaction assays Rubber properties
 In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
chain to another. These links may take the form of covalent bonds or ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
s and the polymers can be either synthetic polymers or natural polymers (such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s).
In polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are a ...
"cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties.
When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interaction
Protein–protein interactions (PPIs) are physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by interactions that include electrostatic forces, hydrogen bonding and th ...
s, as well as other creative cross-linking methodologies.
Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties.
Polymer chemistry
Crosslinking is the general term for the process of forming covalent bonds or relatively short sequences of chemical bonds to join two polymer chains together. The term '' curing'' refers to the crosslinking ofthermosetting
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
resins, such as unsaturated polyester and epoxy resin, and the term ''vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
'' is characteristically used for rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, an ...
s. When polymer chains are crosslinked, the material becomes more rigid.
In polymer chemistry, when a synthetic polymer is said to be "cross-linked", it usually means that the entire bulk of the polymer has been exposed to the cross-linking method. The resulting modification of mechanical properties depends strongly on the cross-link density. Low cross-link densities increase the viscosities of polymer melts. Intermediate cross-link densities transform gummy polymers into materials that have elastomeric properties and potentially high strengths. Very high cross-link densities can cause materials to become very rigid or glassy, such as phenol-formaldehyde materials.

Formation
Cross-links can be formed bychemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s that are initiated by heat, pressure, change in pH, or irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve ...
. For example, mixing of an unpolymerized or partially polymerized resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on n ...
with specific chemicals called crosslinking reagents results in a chemical reaction that forms cross-links. Cross-linking can also be induced in materials that are normally thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains associate ...
through exposure to a radiation source, such as electron beam exposure, gamma radiation, or UV light. For example, electron beam processing
Electron-beam processing or electron irradiation (EBI) is a process that involves using electrons, usually of high energy, to treat an object for a variety of purposes. This may take place under elevated temperatures and nitrogen atmosphere. Poss ...
is used to cross-link the C type of cross-linked polyethylene
Cross-linked polyethylene, commonly abbreviated PEX, XPE or XLPE, is a form of polyethylene with cross-links. It is used predominantly in building services pipework systems, hydronic radiant heating and cooling systems, domestic water piping, ins ...
. Other types of cross-linked polyethylene are made by addition of peroxide during extruding (type A) or by addition of a cross-linking agent (e.g. vinylsilane) and a catalyst during extruding and then performing a post-extrusion curing.
The chemical process of vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
is a type of cross-linking that changes rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, an ...
to the hard, durable material associated with car and bike tire
A tire (American English) or tyre (British English) is a ring-shaped component that surrounds a wheel's rim to transfer a vehicle's load from the axle through the wheel to the ground and to provide traction on the surface over which t ...
s. This process is often called sulfur curing; the term vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
comes from Vulcan
Vulcan may refer to:
Mythology
* Vulcan (mythology), the god of fire, volcanoes, metalworking, and the forge in Roman mythology
Arts, entertainment and media Film and television
* Vulcan (''Star Trek''), name of a fictional race and their home p ...
, the Roman god of fire. This is, however, a slower process. A typical car tire is cured for 15 minutes at 150 °C. However, the time can be reduced by the addition of accelerators such as 2-benzothiazolethiol or tetramethylthiuram disulfide. Both of these contain a sulfur atom in the molecule that initiates the reaction of the sulfur chains with the rubber. Accelerators increase the rate of cure by catalysing the addition of sulfur chains to the rubber molecules.
Cross-links are the characteristic property of thermosetting plastic
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
materials. In most cases, cross-linking is irreversible, and the resulting thermosetting material will degrade or burn if heated, without melting. Especially in the case of commercially used plastics, once a substance is cross-linked, the product is very hard or impossible to recycle. In some cases, though, if the cross-link bonds are sufficiently different, chemically, from the bonds forming the polymers, the process can be reversed. Permanent wave solutions, for example, break and re-form naturally occurring cross-links (disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s) between protein chains in hair.
Physical cross-links
Where chemical cross-links are covalent bonds, physical cross-links are formed by weak interactions. For example, sodiumalginate
Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. With metals such as sodium and calcium, its salts are known as alginates. Its colour ...
gels upon exposure to calcium ion, which allows it to form ionic bonds that bridge between alginate chains. Polyvinyl alcohol gels upon the addition of borax
Borax is a salt ( ionic compound), a hydrated borate of sodium, with chemical formula often written . It is a colorless crystalline solid, that dissolves in water to make a basic solution. It is commonly available in powder or granular for ...
through hydrogen bonding between boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolve ...
and the polymer's alcohol groups. Other examples of materials which form physically cross-linked gels include gelatin, collagen, agarose
Agarose is a heteropolysaccharide, generally extracted from certain red seaweed. It is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is ...
, and agar agar
Agar ( or ), or agar-agar, is a jelly-like substance consisting of polysaccharides obtained from the cell walls of some species of red algae, primarily from ogonori (''Gracilaria'') and "tengusa" (''Gelidiaceae''). As found in nature, agar is ...
.
Chemical covalent cross-links are stable mechanically and thermally, so once formed are difficult to break. Therefore, cross-linked products like car tire
A tire (American English) or tyre (British English) is a ring-shaped component that surrounds a wheel's rim to transfer a vehicle's load from the axle through the wheel to the ground and to provide traction on the surface over which t ...
s cannot be recycled easily. A class of polymers known as thermoplastic elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers, are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric properties. ...
s rely on physical cross-links in their microstructure to achieve stability, and are widely used in non-tire applications, such as snowmobile
A snowmobile, also known as a Ski-Doo, snowmachine, sled, motor sled, motor sledge, skimobile, or snow scooter, is a motorized vehicle designed for winter travel and recreation on snow. It is designed to be operated on snow and ice and does not ...
tracks, and catheter
In medicine, a catheter (/ˈkæθətər/) is a thin tubing (material), tube made from medical grade materials serving a broad range of functions. Catheters are medical devices that can be inserted in the body to treat diseases or perform a surgi ...
s for medical use. They offer a much wider range of properties than conventional cross-linked elastomers because the domains that act as cross-links are reversible, so can be reformed by heat. The stabilizing domains may be non-crystalline (as in styrene-butadiene block copolymers) or crystalline as in thermoplastic copolyesters.
Oxidative cross-links
Many polymers undergo oxidative cross-linking, typically when exposed to atmospheric oxygen. In some cases this is undesirable and thus polymerization reactions may involve the use of an antioxidant to slow the formation of oxidative cross-links. In other cases, when formation of cross-links by oxidation is desirable, an oxidizer such as hydrogen peroxide may be used to speed up the process. The aforementioned process of applying a permanent wave to hair is one example of oxidative cross-linking. In that process the disulfide bonds are reduced, typically using a mercaptan such as ammonium thioglycolate. Following this, the hair is curled and then "neutralized". The neutralizer is typically an acidic solution of hydrogen peroxide, which causes new disulfide bonds to form under conditions of oxidation, thus permanently fixing the hair into its new configuration. It also happens forgluten
Gluten is a structural protein naturally found in certain cereal grains. Although "gluten" often only refers to wheat proteins, in medical literature it refers to the combination of prolamin and glutelin proteins naturally occurring in all grai ...
which change structure of foods.
In biology
Proteins naturally present in the body can contain crosslinks generated byenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
-catalyzed or spontaneous reactions. Such crosslinks are important in generating mechanically stable structures such as hair, skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other animal coverings, such as the arthropod exoskeleton, have different de ...
, and cartilage. Disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
formation is one of the most common crosslinks, but isopeptide bond formation is also common. Proteins can also be cross-linked artificially using small-molecule crosslinkers. Compromised collagen in the cornea, a condition known as keratoconus, can be treated with clinical crosslinking.
In biological context crosslinking could play a role in atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis in which the wall of the artery develops abnormalities, called lesions. These lesions may lead to narrowing due to the buildup of atheromatous plaque. At onset there are usually no s ...
through advanced glycation end-product
Advanced glycation end products (AGEs) are proteins or lipids that become glycated as a result of exposure to sugars. They are a bio-marker implicated in aging and the development, or worsening, of many degenerative diseases, such as diabetes, ath ...
s (AGEs), which have been implicated to induce crosslinking of collagen, which may lead to vascular stiffening.
Use in protein study
The interactions or mere proximity ofprotein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s can be studied by the clever use of crosslinking agents. For example, protein A and protein B may be very close to each other in a cell, and a chemical crosslinker could be used to probe the protein–protein interaction
Protein–protein interactions (PPIs) are physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by interactions that include electrostatic forces, hydrogen bonding and th ...
between these two proteins by linking them together, disrupting the cell, and looking for the crosslinked proteins.
A variety of crosslinkers are used to analyze subunit structure of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, protein interactions, and various parameters of protein function by using differing crosslinkers, often with diverse spacer arm lengths. Subunit structure is deduced, since crosslinkers bind only surface residues in relatively close proximity in the native state. Protein interactions are often too weak or transient to be easily detected, but by crosslinking, the interactions can be stabilized, captured, and analyzed.
Examples of some common crosslinkers are the imidoester crosslinker dimethyl suberimidate, the N-Hydroxysuccinimide-ester crosslinker BS3 and formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
. Each of these crosslinkers induces nucleophilic attack of the amino group of lysine and subsequent covalent bonding via the crosslinker. The zero-length carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesi ...
crosslinker EDC functions by converting carboxyls into amine-reactive isourea intermediates that bind to lysine residues or other available primary amines. SMCC or its water-soluble analog, Sulfo-SMCC, is commonly used to prepare antibody-hapten conjugates for antibody development.
''In-vitro'' cross-linking method, termed PICUP ( photo-induced cross-linking of unmodified proteins), was developed in 1999. They devised a process in which ammonium persulfate
Ammonium persulfate (APS) is the inorganic compound with the formula (NH4)2S2O8. It is a colourless (white) salt that is highly soluble in water, much more so than the related potassium salt. It is a strong oxidizing agent that is used as a catalys ...
(APS), which acts as an electron acceptor, and tris(bipyridine)ruthenium(II) chloride, tris-bipyridylruthenium (II) cation () are added to the protein of interest and irradiated with UV light. PICUP is more expeditious and high yielding compared to previous chemical cross-linking methods.
''In-vivo'' crosslinking of protein complexes using photo-reactive amino acid analogs was introduced in 2005 by researchers from the Max Planck Institute of Molecular Cell Biology and Genetics. In this method, cells are grown with photoreactive diazirine analogs to leucine and methionine, which are incorporated into proteins. Upon exposure to ultraviolet light, the diazirines are activated and bind to interacting proteins that are within a few ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
s of the photo-reactive amino acid analog (UV cross-linking).
In material science
Wide usage of the term cross-linking is also described as vulcanization in material science and engineering, mainly addressing the fast reaction between monomers and polymers and branches of rubber. Cross-linking or interlinking m of polymers can improve the mechanical properties of the polymeric material and also can improve the adhesion between two interfaces of the coating.Promote the mechanical properties of material
Cross-linked polymers, monomers, and branches can improve the bulk mechanical properties of the material and mostly decrease the viscosity of non-solid materials. The earliest examples of crosslinking, linking long chains of polymers together to increase strength and mass, involved tires. Rubber was vulcanized with sulfur under heat, which created a link between latex models. Synthetically crosslinked polymers have many uses, including those in the biological sciences, such as applications in forming polyacrylamide gels for gel electrophoresis. Synthetic rubber used fortire
A tire (American English) or tyre (British English) is a ring-shaped component that surrounds a wheel's rim to transfer a vehicle's load from the axle through the wheel to the ground and to provide traction on the surface over which t ...
s is made by crosslinking rubber through the process of vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
. This crosslinking makes them more elastic. Hard-shell kayaks are also often manufactured with crosslinked polymers.
In many hydraulic fracturing treatments, a delayed gel-cross-linker fluid is used to carry out fracture treatment of the rock.
Promote the adhesion of coatings
Two interlinked layers of the interface can increase the adhesion strength of the coating to the surface. Other examples of polymers that can be crosslinked are ethylene-vinyl acetate – as used in solar panel manufacturing – andpolyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including b ...
.
Alkyd enamels, the dominant type of commercial oil-based paint, cure by oxidative crosslinking after exposure to air.
Measuring degree of crosslinking
Crosslinking is often measured by swelling tests. The crosslinked sample is placed into a good solvent at a specific temperature, and either the change in mass or the change in volume is measured. The more crosslinking, the less swelling is attainable. Based on the degree of swelling, the Flory Interaction Parameter (which relates the solvent interaction with the sample), and the density of the solvent, the theoretical degree of crosslinking can be calculated according to Flory's Network Theory. Two ASTM standards are commonly used to describe the degree of crosslinking in thermoplastics. In ASTM D2765, the sample is weighed, then placed in a solvent for 24 hours, weighed again while swollen, then dried and weighed a final time. The degree of swelling and the soluble portion can be calculated. In another ASTM standard, F2214, the sample is placed in an instrument that measures the height change in the sample, allowing the user to measure the volume change. The crosslink density can then be calculated.See also
* Branching (polymer chemistry) * Cross-linked enzyme aggregate *Cross-linked polyethylene
Cross-linked polyethylene, commonly abbreviated PEX, XPE or XLPE, is a form of polyethylene with cross-links. It is used predominantly in building services pipework systems, hydronic radiant heating and cooling systems, domestic water piping, ins ...
(PEX)
* Crosslinking of DNA
* Fixation (histology)
*Phenol formaldehyde resin
Phenol formaldehyde resins (PF) or phenolic resins (also infrequently called phenoplasts) are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commerc ...
(phenolic resin)
References
{{Reflist, 30emExternal links
Application note on how to measure degree of crosslinking in plastics
Ageing processes Polymer chemistry Protein–protein interaction assays Rubber properties