Corannulene on:
[Wikipedia]
[Google]
[Amazon]
Corannulene is a
 The bromine substituents are removed with an excess of ''n''-butyllithium.
A kilogram scale synthesis of corannulene has been achieved.
Much effort is directed at functionalization of the corannulene ring with novel functional groups such as ethynyl groups, ether groups, thioether groups, platinum functional groups, aryl groups, phenalenyl fused and indeno extensions. and ferrocene groups.
The bromine substituents are removed with an excess of ''n''-butyllithium.
A kilogram scale synthesis of corannulene has been achieved.
Much effort is directed at functionalization of the corannulene ring with novel functional groups such as ethynyl groups, ether groups, thioether groups, platinum functional groups, aryl groups, phenalenyl fused and indeno extensions. and ferrocene groups.
 However, later theoretical calculations have disputed the validity of this approximation.
However, later theoretical calculations have disputed the validity of this approximation.
 The corannulene group is used in
The corannulene group is used in
polycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. ...
with chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
C20 H10. The molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
consists of a cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occu ...
ring fused with 5 benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
rings, so another name for it is irculene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded ...
. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal
The calorie is a unit of energy. For historical reasons, two main definitions of "calorie" are in wide use. The large calorie, food calorie, or kilogram calorie was originally defined as the amount of heat needed to raise the temperature of o ...
/ mol (42.7 kJ/mol) at −64 °C.
Synthesis
Several synthetic routes exist to corannulene.Flash vacuum pyrolysis Flash vacuum pyrolysis (FVP) is a technique in organic synthesis. It entails heating a precursor molecule intensely and briefly. Two key parameters are the temperature and duration (or residence time), which are adjusted to optimize yield, conver ...
techniques generally have lower chemical yield
In chemistry, yield, also referred to as reaction yield, is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage. Yield is one of the pr ...
s than solution-chemistry syntheses, but offer routes to more derivatives. Corannulane was first isolated in 1966 by multistep organic synthesis. In 1971, the synthesis and properties of corannulane were reported. A flash vacuum pyrolysis method followed in 1991. One synthesis based on solution chemistry consists of a nucleophilic displacement
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
–elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
of an octabromide with sodium hydroxide:
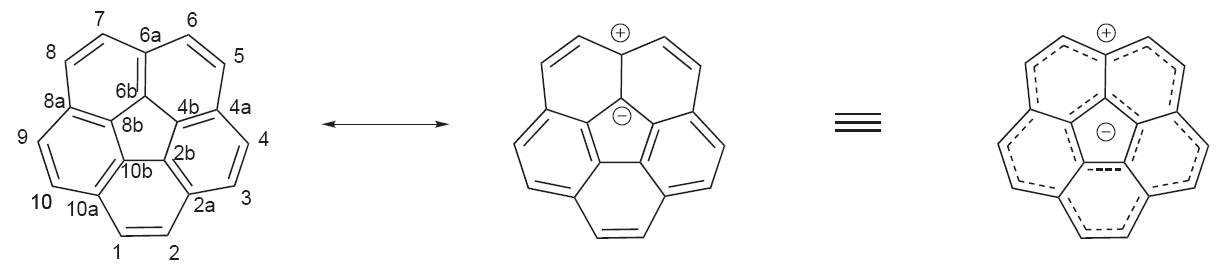
:Aromaticity
The observedaromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
for this compound is explained with a so-called annulene-within-an-annulene model. According to this model corannulene is made up of an aromatic 6 electron cyclopentadienyl anion
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of and abbreviated as Cp−. It is formed from the deprotonation of the molecule cyclopentadiene.
Properties
The cyclopentadienyl anion i ...
surrounded by an aromatic 14 electron annulenyl cation. This model was suggested by Barth and Lawton in the first synthesis of corannulene in 1966. They also suggested the trivial name 'corannulene', which is derived from the annulene-within-an-annulene model: core + annulene.
: However, later theoretical calculations have disputed the validity of this approximation.
However, later theoretical calculations have disputed the validity of this approximation.
Reactions
Reduction
Corannulene can be reduced up to a tetraanion in a series ofone-electron reduction
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
s. This has been performed with alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
, electrochemically and with bases. The corannulene dianion is antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aroma ...
and tetraanion is again aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
. With lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
as reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth me ...
two tetraanions form a supramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
dimer with two bowls stacked into each other with 4 lithium ions in between and 2 pairs above and below the stack. This self-assembly motif was applied in the organization of fullerenes. Penta-substituted fullerenes (with methyl or phenyl groups) charged with five electrons form supramolecular dimers with a complementary corannulene tetraanion bowl, 'stitched' by interstitial lithium cations. In a related system 5 lithium ions are sandwiched between two corannulene bowls
In one cyclopenta corannulene a concave - concave aggregate is observed by NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
with 2 C–Li–C bonds connecting the tetraanions.
:
Metals tend to bind to the convex face of the annulene. Concave binding has been reported for a cesium / crown ether system
Oxidation
UV 193-nm photoionization effectively removes a π-electron from the twofold degenerate E1-HOMO located in the aromatic network of electrons yielding a corannulene radical cation. Owing to the degeneracy in the HOMO orbital, the corannulene radical cation is unstable in its original C5v molecular arrangement, and therefore, subject to Jahn-Teller (JT) vibronic distortion. Using electrospray ionization, a protonated corannulene cation has been produced in which the protonation site was observed to be on a peripheral sp2-carbon atom.Reaction with electrophiles
Corannulene can react withelectrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
s to form a corannulene carbocation. Reaction with chloromethane
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
and aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
results in the formation of an AlCl4− salt with a methyl group situated at the center with the cationic center at the rim. X-ray diffraction analysis shows that the new carbon-carbon bond is elongated (157 pm)
Bicorannulenyl
Bicorannulenyl is the product of dehydrogenative coupling of corannulene. With the formula C20H9-C20H9, it consists of two corannulene units connected through a single C-C bond. The molecule's stereochemistry consists of two chiral elements: the asymmetry of a singly substituted corannulenyl, and the helical twist about the central bond. In the neutral state, bicorannulenyl exists as 12 conformers, which interconvert through multiple bowl-inversions and bond-rotations. When bicorannulenyl is reduced to a dianion with potassium metal, the central bond assumes significant double-bond character. This change is attributed to the orbital structure, which has a LUMO orbital localized on the central bond. When bicorannulenyl is reduced to an octaanion with lithium metal, it self-assembles into supramolecular oligomers. This motif illustrates "charged polyarene stacking".Research
 The corannulene group is used in
The corannulene group is used in host–guest chemistry In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest ch ...
with interactions based on pi stacking, notably with fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
s (the buckycatcher) but also with nitrobenzene
Alkyl-substituted corannulenes form a thermotropic hexagonal columnar liquid crystalline mesophase. Corannulene has also been used as the core group in a dendrimer
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The ...
. Like other PAHs, corannulene ligates metals. Corannulenes with ethynyl groups are investigated for their potential use as blue emitters. The structure was analyzed by infrared spectroscopy, Raman spectroscopy, and X-ray photoelectron spectroscopy.
See also
*Coronene
Coronene (also known as superbenzene and cyclobenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is . It is a yellow material that dissolves in common solvents including benzene, tol ...
* Helicene
In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules. The chemistry of helicenes has attracted continuin ...
* Geodesic polyarene
References
{{PAHs Geodesic polyarenes Polycyclic aromatic hydrocarbons