Copper nanoparticle on:
[Wikipedia]
[Google]
[Amazon]
A copper nanoparticle is a copper based particle 1 to 100 nm in size.Khan, F.A. ''Biotechnology Fundamentals''; CRC Press; Boca Raton, 2011 Like many other forms of
 One of the earliest uses of copper nanoparticles was to color glass and
One of the earliest uses of copper nanoparticles was to color glass and
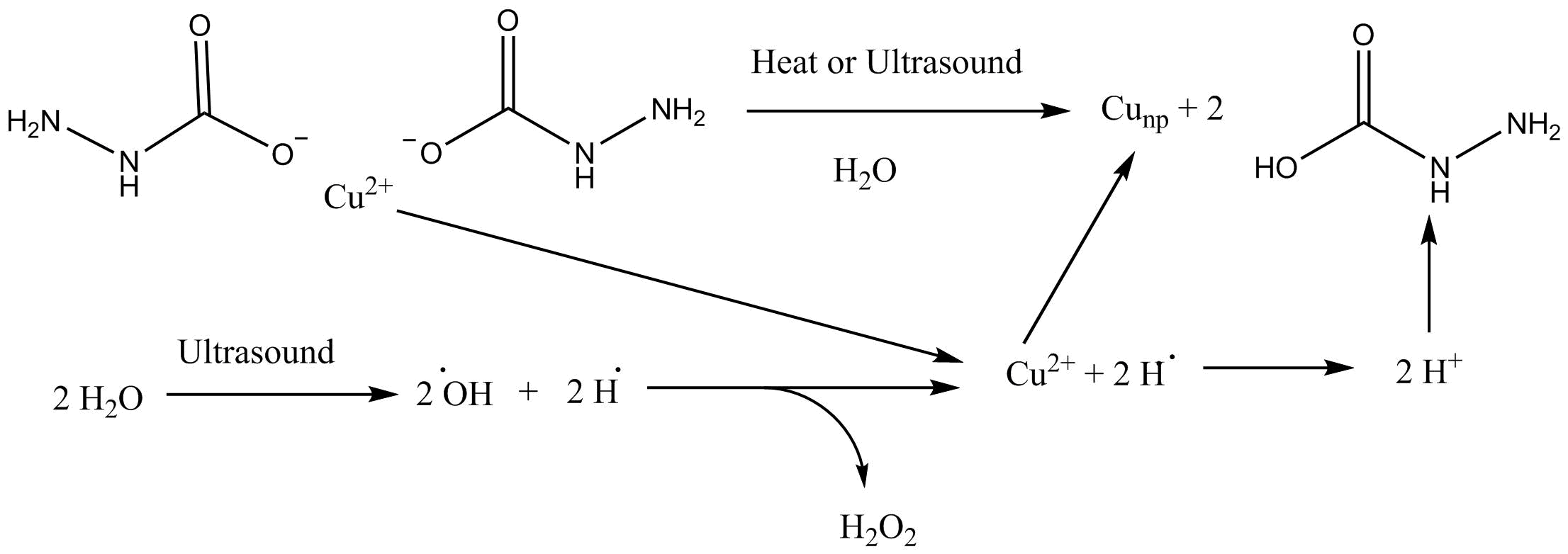 Various methods have been described to chemically synthesize copper nanoparticles. An older method involves the reduction of copper hydrazine carboxylate in an aqueous solution using reflux or by heating through
Various methods have been described to chemically synthesize copper nanoparticles. An older method involves the reduction of copper hydrazine carboxylate in an aqueous solution using reflux or by heating through
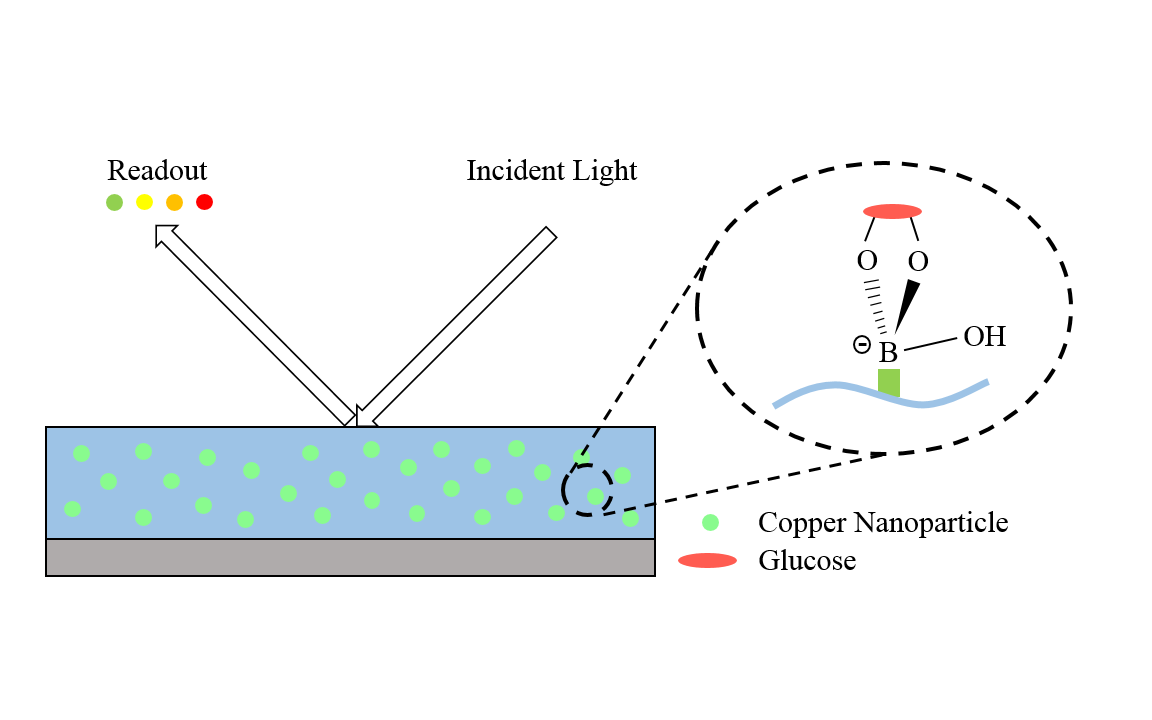 One of the examples is a glucose sensor. With the use of copper nanoparticles, the sensor does not require any enzyme and therefore has no need to deal with enzyme degradation and denaturation. As described in Figure 3, depending on the level of glucose, the nanoparticles in the sensor diffract the incident light at a different angle. Consequently, the resulting diffracted light gives a different color based on the level of glucose. In fact, the nanoparticles enable the sensor to be more stable at high temperatures and varying pH, and more resistant to toxic chemicals. Moreover, using nanoparticles, native amino acids can be detected. A copper nanoparticle-plated screen-printed carbon electrode functions as a stable and effective sensing system for all 20 amino acid detection.
One of the examples is a glucose sensor. With the use of copper nanoparticles, the sensor does not require any enzyme and therefore has no need to deal with enzyme degradation and denaturation. As described in Figure 3, depending on the level of glucose, the nanoparticles in the sensor diffract the incident light at a different angle. Consequently, the resulting diffracted light gives a different color based on the level of glucose. In fact, the nanoparticles enable the sensor to be more stable at high temperatures and varying pH, and more resistant to toxic chemicals. Moreover, using nanoparticles, native amino acids can be detected. A copper nanoparticle-plated screen-printed carbon electrode functions as a stable and effective sensing system for all 20 amino acid detection.
nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
s, a copper nanoparticle can be prepared by natural processes or through chemical synthesis. These nanoparticles are of particular interest due to their historical application as coloring agents and the biomedical as well as the antimicrobial
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals ar ...
ones.
Historical uses
 One of the earliest uses of copper nanoparticles was to color glass and
One of the earliest uses of copper nanoparticles was to color glass and ceramics
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain ...
during the ninth century in Mesopotamia
Mesopotamia ''Mesopotamíā''; ar, بِلَاد ٱلرَّافِدَيْن or ; syc, ܐܪܡ ܢܗܪ̈ܝܢ, or , ) is a historical region of Western Asia situated within the Tigris–Euphrates river system, in the northern part of the ...
. This was done by creating a glaze with copper and silver salts and applying it to clay pottery. When the pottery was baked at high temperatures in reducing conditions, the metal ions migrated to the outer part of the glaze and were reduced to metals. The end result was a double layer of metal nanoparticles with a small amount of glaze in between them. When the finished pottery was exposed to light, the light would penetrate and reflect off the first layer. The light penetrating the first layer would reflect off the second layer of nanoparticles and cause interference
Interference is the act of interfering, invading, or poaching. Interference may also refer to:
Communications
* Interference (communication), anything which alters, modifies, or disrupts a message
* Adjacent-channel interference, caused by extr ...
effects with light reflecting off the first layer, creating a luster effect that results from both constructive and destructive interference.
Synthesis
 Various methods have been described to chemically synthesize copper nanoparticles. An older method involves the reduction of copper hydrazine carboxylate in an aqueous solution using reflux or by heating through
Various methods have been described to chemically synthesize copper nanoparticles. An older method involves the reduction of copper hydrazine carboxylate in an aqueous solution using reflux or by heating through ultrasound
Ultrasound is sound waves with frequencies higher than the upper audible limit of human hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hear it. This limit varies ...
under an inert argon atmosphere. This results in a combination of copper oxide and pure copper nanoparticle clusters, depending on the method used. A more modern synthesis utilizes copper(II) chloride
Copper(II) chloride is the chemical compound with the chemical formula CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.
Both the anhydrous and the dihydrate forms occur naturally as the ver ...
in a room temperature reaction with sodium citrate Sodium citrate may refer to any of the sodium salts of citric acid (though most commonly the third):
* Monosodium citrate
* Disodium citrate
* Trisodium citrate
The three forms of salt are collectively known by the E number E331.
Applications ...
or myristic acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nut ...
in an aqueous solution containing sodium formaldehyde sulfoxylate to obtain a pure copper nanoparticle powder. While these syntheses generate fairly consistent copper nanoparticles, the possibility of controlling the sizes and shapes of copper nanoparticles has also been reported. The reduction of copper(II) acetylacetonate in organic solvent with oleyl amine and oleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omeg ...
causes the formation of rod and cube-shaped nanoparticles while variations in reaction temperature affect the size of the synthesized particles.
Another method of synthesis involves using copper (II) hydrazine carboxylate salt with ultrasound or heat in water to generate a radical reaction, as shown in the figure to the right. Copper nanoparticles can also be synthesized using green chemistry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental che ...
to reduce the environmental impact of the reaction. Copper chloride can be reduced using only L-ascorbic acid in a heated aqueous solution to produce stable copper nanoparticles.
Characteristics
Copper nanoparticles display unique characteristics including catalytic and antifungal/antibacterial activities that are not observed in commercial copper. First of all, copper nanoparticles demonstrate a very strong catalytic activity, a property that can be attributed to their large catalytic surface area. With the small size and great porosity, the nanoparticles are able to achieve a higher reaction yield and a shorter reaction time when utilized as reagents in organic and organometallic synthesis. In fact, copper nanoparticles that are used in a condensation reaction of iodobenzene attained about 88% conversion to biphenyl, while the commercial copper exhibited only a conversion of 43%. Copper nanoparticles that are extremely small and have a high surface to volume ratio can also serve as antifungal/antibacterial agents. The antimicrobial activity is induced by their close interaction with microbial membranes and their metal ions released in solutions. As the nanoparticles oxidize slowly in solutions, cupric ions are released from them and they can create toxic hydroxyl free radicals when the lipid membrane is nearby. Then, the free radicals disassemble lipids in cell membranes through oxidation to degenerate the membranes. As a result, the intracellular substances seep out of cells through the destructed membranes; the cells are no longer able to sustain fundamental biochemical processes. In the end, all these alterations inside of the cell caused by the free radicals lead to cell death.Applications
Copper nanoparticles with great catalytic activities can be applied to biosensors and electrochemical sensors. Redox reactions utilized in those sensors are generally irreversible and also require high overpotentials (more energy) to run. In fact, the nanoparticles have the ability to make the redox reactions reversible and to lower the overpotentials when applied to the sensors. One of the examples is a glucose sensor. With the use of copper nanoparticles, the sensor does not require any enzyme and therefore has no need to deal with enzyme degradation and denaturation. As described in Figure 3, depending on the level of glucose, the nanoparticles in the sensor diffract the incident light at a different angle. Consequently, the resulting diffracted light gives a different color based on the level of glucose. In fact, the nanoparticles enable the sensor to be more stable at high temperatures and varying pH, and more resistant to toxic chemicals. Moreover, using nanoparticles, native amino acids can be detected. A copper nanoparticle-plated screen-printed carbon electrode functions as a stable and effective sensing system for all 20 amino acid detection.
One of the examples is a glucose sensor. With the use of copper nanoparticles, the sensor does not require any enzyme and therefore has no need to deal with enzyme degradation and denaturation. As described in Figure 3, depending on the level of glucose, the nanoparticles in the sensor diffract the incident light at a different angle. Consequently, the resulting diffracted light gives a different color based on the level of glucose. In fact, the nanoparticles enable the sensor to be more stable at high temperatures and varying pH, and more resistant to toxic chemicals. Moreover, using nanoparticles, native amino acids can be detected. A copper nanoparticle-plated screen-printed carbon electrode functions as a stable and effective sensing system for all 20 amino acid detection.
References
{{Reflist Copper Nanoparticles by composition