Compounds of palladium(III) on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, compounds of palladium(III) feature the noble metal
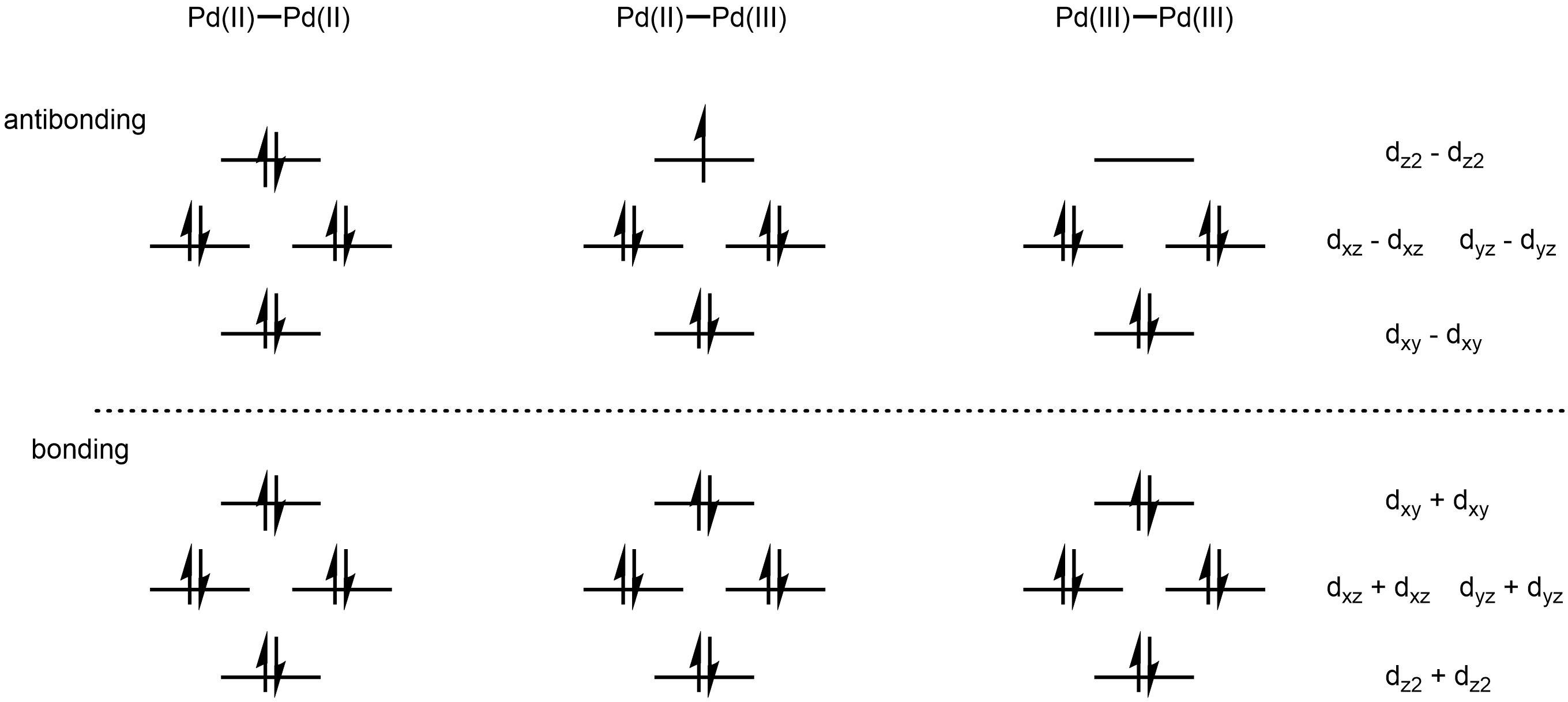 The first example of a dipalladium(III) complex was obtained by oxidation of dinuclear Pd(II) complex of
The first example of a dipalladium(III) complex was obtained by oxidation of dinuclear Pd(II) complex of
palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
in the unusual +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
(in most of its compounds, palladium has the oxidation state II). Compounds of Pd(III) occur in mononuclear and dinuclear forms. Palladium(III) is most often invoked, not observed in mechanistic organometallic chemistry.
Mononuclear compounds
Pd(III) has a d7electronic configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
, which leads to a Jahn-Teller distorted octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet a ...
geometry. The geometry could also be viewed as being intermediate between square-planar
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corn ...
and octahedral. These complexes are low-spin and paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
.
The first Pd(III) complex characterized by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
was reported in 1987. It was obtained by oxidation of the 1,4,7-trithiacyclononane
1,4,7-Trithiacyclononane, also called 9-ane-S3, is the thia-crown ether with the formula (CH2CH2S)3. This cyclic thioether is most often encountered as a tridentate ligand in coordination chemistry, where it forms transition metal thioether com ...
(ttcn) complex d(ttcn)2sup>3+. X-Ray crystallography revealed the expected Jahn-Teller distorted octahedral geometry, in spite of the highly symmetric structure of the ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
.
The first organometallic Pd(III) complex characterized by X-Ray crystallography was reported in 2010. Organopalladium complexes supported with a macrocyclic tetradentate ligand undergo single-electron oxidation to give Pd(III) species that is stabilized by the axially-positioned amine. The authors propose that while the axial nitrogen stabilize a distorted octahedral geometry, the t-Bu group and the rigidity of the macrocyclic
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
structure inhibits the oxidation to a more conventional octahedral Pd(IV).
Dinuclear compounds
Structure
Pairs of Pd(III) centers can couple, giving rise to a Pd–Pd bond order of 1. A two-electron oxidation of two Pd(II) species can generate a diamagnetic, Pd(III)-Pd(III)dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
with a bond order of 1.
 The first example of a dipalladium(III) complex was obtained by oxidation of dinuclear Pd(II) complex of
The first example of a dipalladium(III) complex was obtained by oxidation of dinuclear Pd(II) complex of triazabicyclodecene
Triazabicyclodecene (1,5,7-triazabicyclo .4.0ec-5-ene or TBD) is an organic compound consisting of a bicyclic guanidine. For a charge-neutral compound, it is a relatively strong base that is effectively for a variety of organic transformations. ...
.
The first organometallic dinuclear Pd(III) complexes were reported in 2006 by Cotton and coworkers as well. These complexes catalyze the diborylation of terminal olefins. Due to the facile reduction of these complexes to Pd(II) species by diborane, the authors proposed that the dinuclear Pd(III) complexes serve as precatalysts for active Pd(II) catalysts.Reactivity
The reactivity of dinuclear Pd(III) species as active catalytic intermediate is mostly discussed in the context of C-H activation. While it was proposed that Pd-catalyzed oxidative C-H functionalization reactions involve a Pd(IV) intermediate, Ritter and coworkers first postulated that these oxidative reactions could involve a dinuclear Pd(III) intermediate instead of Pd(IV). Dinuclear Pd species are involved in Pd-catalyzed C-H chlorination. Through X-ray crystallography, Ritter unambiguously showed that dinuclear Pd(III) complex is formed when the palladacycle is treated with two-electron oxidant, and such dinuclear complex undergoes C-Cl reductive elimination under ambient temperature. Both experimental and computational data was consistent with a concerted 1,1-reductive elimination mechanism for the C-Cl forming step. The authors show that such bimetallic participation of redox event lowers the activation barrier for reductive elimination step by ~30 kcal/mol compared to a monometallic pathway.Acetoxylation
:
In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposit ...
of 2-phenylpyridine
2-Phenylpyridine is an organic compound with the formula C6H5C5H4N (or C11H9N). It is a colourless viscous liquid. The compound and related derivatives have attracted interest as precursors to highly fluorescent metal complexes of possible value ...
was also demonstrated to involve a dinuclear Pd(III) intermediate.
References
{{Chemical compounds by element Palladium compounds P