Chirality (chemistry) on:
[Wikipedia]
[Google]
[Amazon]

 In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations,
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations,
 While the presence of a stereogenic center describes the great majority of chiral molecules, many variations and exceptions exist. For instance it is not necessary for the chiral substance to have a stereogenic center. Examples include 1-bromo-3-chloro-5-fluoro
While the presence of a stereogenic center describes the great majority of chiral molecules, many variations and exceptions exist. For instance it is not necessary for the chiral substance to have a stereogenic center. Examples include 1-bromo-3-chloro-5-fluoro
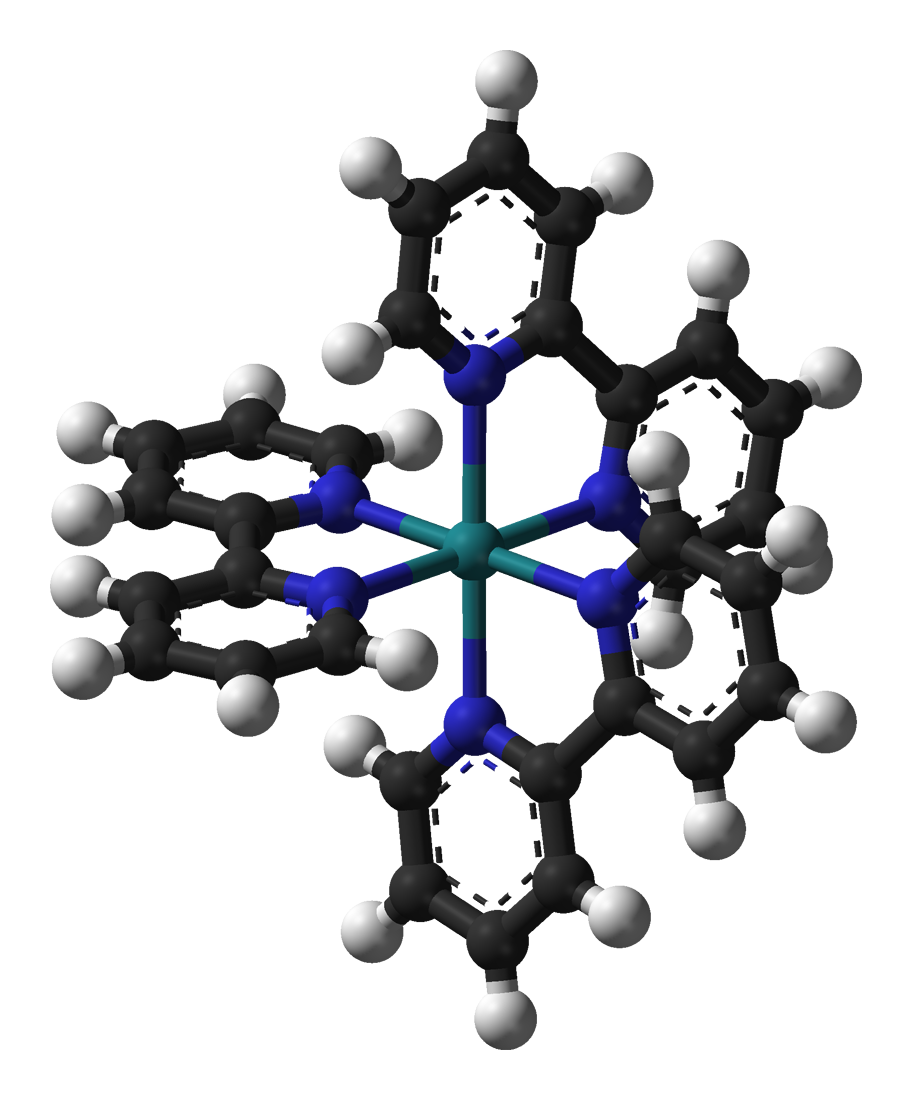 Chirality is a symmetry property, not a property of any part of the periodic table. Thus many inorganic materials, molecules, and ions are chiral.
Chirality is a symmetry property, not a property of any part of the periodic table. Thus many inorganic materials, molecules, and ions are chiral.
21st International Symposium on Chirality
* ttps://web.archive.org/web/20071226005203/http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA3t5.html IUPAC nomenclature for amino acid configurations.
Michigan State University's explanation of R/S nomenclatureChirality & Bioactivity I.: Pharmacology
Chirality and the Search for Extraterrestrial Life
* The Handedness of the Universe by Roger A Hegstrom and Dilip K Kondepudi http://quantummechanics.ucsd.edu/ph87/ScientificAmerican/Sciam/Hegstrom_The_Handedness_of_the_universe.pdf {{DEFAULTSORT:Chirality (Chemistry) Stereochemistry Polarization (waves) Chirality Chemical nomenclature Biochemistry Origin of life Pharmacology
 In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations,
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translation
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
s, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property.
A chiral molecule or ion exists in two stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration
Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity (or group) and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonde ...
or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
, and it usually differs chemically and physically from the pure enantiomers.
Chiral molecules will usually have a stereogenic element from which chirality arises. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. A given stereocenter has two possible configurations, which give rise to stereoisomers (diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
and enantiomers) in molecules with one or more stereocenter. For a chiral molecule with one or more stereocenter, the enantiomer corresponds to the stereoisomer in which every stereocenter has the opposite configuration. An organic compound with only one stereogenic carbon is always chiral. On the other hand, an organic compound with multiple stereogenic carbons is typically, but not always, chiral. In particular, if the stereocenters are configured in such a way that the molecule has an internal plane of symmetry, then the molecule is achiral and is known as a ''meso'' compound. Less commonly, other atoms like N, P, S, and Si can also serve as stereocenters, provided they have four distinct substituents (including lone pair electrons) attached to them.
Molecules with chirality arising from one or more stereocenters are classified as possessing central chirality. There are two other types of stereogenic elements that can give rise to chirality, a stereogenic axis ( axial chirality) and a stereogenic plane ( planar chirality). Finally, the inherent curvature of a molecule can also give rise to chirality ( inherent chirality). These types of chirality are far less common than central chirality. BINOL is a typical example of an axially chiral molecule, while ''trans''-cyclooctene is a commonly cited example of a planar chiral molecule. Finally, helicene
In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules. The chemistry of helicenes has attracted continuin ...
possesses helical chirality, which is one type of inherent chirality.
Chirality is an important concept for stereochemistry and biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
. Most substances relevant to biology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary i ...
are chiral, such as carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
s (sugars
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
, starch, and cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
), the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s that are the building blocks of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, and the nucleic acids. In living organisms, one typically finds only one of the two enantiomers of a chiral compound. For that reason, organisms that consume a chiral compound usually can metabolize only one of its enantiomers. For the same reason, the two enantiomers of a chiral pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field an ...
usually have vastly different potencies or effects.
Definition
The chirality of a molecule is based on the molecular symmetry of its conformations. A conformation of a molecule is chiral if and only if it belongs to the ''Cn'', ''Dn'', ''T'', ''O'', ''I''point groups
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every ...
(the chiral point groups). However, whether the molecule itself is considered to be chiral depends on whether its chiral conformations are persistent isomers that could be isolated as separated enantiomers, at least in principle, or the enantiomeric conformers rapidly interconvert at a given temperature and timescale through low-energy conformational changes (rendering the molecule achiral). For example, despite having chiral ''gauche'' conformers that belong to the ''C''2 point group, butane is considered achiral at room temperature because rotation about the central C–C bond rapidly interconverts the enantiomers (3.4 kcal/mol barrier). Similarly, ''cis''-1,2-dichlorocyclohexane consists of chair conformers that are nonidentical mirror images, but the two can interconvert via the cyclohexane chair flip (~10 kcal/mol barrier). As another example, amines with three distinct substituents (R1R2R3N:) are also regarded as achiral molecules because their enantiomeric pyramidal conformers rapidly invert and interconvert through a planar transition state (~6 kcal/mol barrier).
However, if the temperature in question is low enough, the process that interconverts the enantiomeric chiral conformations becomes slow compared to a given timescale. The molecule would then be considered to be chiral at that temperature. The relevant timescale is, to some degree, arbitrarily defined: 1000 seconds is sometimes employed, as this is regarded as the lower limit for the amount of time required for chemical or chromatographic separation of enantiomers in a practical sense. Molecules that are chiral at room temperature due to restricted rotation about a single bond (barrier to rotation ≥ ca. 23 kcal/mol) are said to exhibit atropisomerism.
A chiral compound can contain no improper axis of rotation (''Sn''), which includes planes of symmetry and inversion center. Chiral molecules are always dissymmetric (lacking ''Sn'') but not always asymmetric (lacking all symmetry elements except the trivial identity). Asymmetric molecules are always chiral.
The following table shows some examples of chiral and achiral molecules, with the Schoenflies notation The Schoenflies (or Schönflies) notation, named after the German mathematician Arthur Moritz Schoenflies, is a notation primarily used to specify point groups in three dimensions. Because a point group alone is completely adequate to describe the ...
of the point group of the molecule. In the achiral molecules, X and Y (with no subscript) represent achiral groups, whereas X and X or Y and Y represent enantiomers. Note that there is no meaning to the orientation of an ''S'' axis, which is just an inversion. Any orientation will do, so long as it passes through the center of inversion. Also note that higher symmetries of chiral and achiral molecules also exist, and symmetries that do not include those in the table, such as the chiral ''C'' or the achiral ''S''.
Stereogenic centers
Many chiral molecules have point chirality, namely a single chiral stereogenic center that coincides with an atom. This stereogenic center usually has four or more bonds to different groups, and may be carbon (as in many biological molecules), phosphorus (as in many organophosphates), silicon, or a metal (as in many chiral coordination compounds). However, a stereogenic center can also be a trivalent atom whose bonds are not in the same plane, such asphosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
in P-chiral phosphines (PRR′R″) and sulfur in S-chiral sulfoxides (OSRR′), because a lone-pair of electrons is present instead of a fourth bond.
Chirality can also arise from isotopic differences between atoms, such as in the deuterated benzyl alcohol
Benzyl alcohol is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid w ...
PhCHDOH; which is chiral and optically active ( 'α''sub>D = 0.715°), even though the non-deuterated compound PhCH2OH is not.
If two enantiomers easily interconvert, the pure enantiomers may be practically impossible to separate, and only the racemic mixture is observable. This is the case, for example, of most amines with three different substituents (NRR′R″), because of the low energy barrier for nitrogen inversion.
 While the presence of a stereogenic center describes the great majority of chiral molecules, many variations and exceptions exist. For instance it is not necessary for the chiral substance to have a stereogenic center. Examples include 1-bromo-3-chloro-5-fluoro
While the presence of a stereogenic center describes the great majority of chiral molecules, many variations and exceptions exist. For instance it is not necessary for the chiral substance to have a stereogenic center. Examples include 1-bromo-3-chloro-5-fluoroadamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the ...
, methylethylphenyl tetrahedrane, certain calixarenes and fullerenes
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
, which have inherent chirality. The C2-symmetric species 1,1′-bi-2-naphthol
1,1′-Bi-2-naphthol (BINOL) is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation ...
(BINOL), 1,3-dichloroallene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which ...
have axial chirality. (''E'')-cyclooctene Cyclooctene is the cycloalkene with a formula . Its molecule has a ring of 8 carbon atoms, connected by seven single bonds and one double bond.
Cyclooctene is notable because it is the smallest cycloalkene that can exist stably as either the cis� ...
and many ferrocenes have planar chirality.
When the optical rotation for an enantiomer is too low for practical measurement, the species is said to exhibit cryptochirality.
Chirality is an intrinsic part of the identity of a molecule, so the systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivial ...
includes details of the absolute configuration
Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity (or group) and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonde ...
(''R/S'', ''D/L'', or other designations).
Manifestations of chirality
* Flavor: theartificial sweetener
A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie () or low-calorie sweetener. Artificial sweeteners may b ...
aspartame has two enantiomers. L-aspartame tastes sweet whereas D-aspartame is tasteless.
* Odor: ''R''-(–)-carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill.
Uses
Both c ...
smells like spearmint
Spearmint, also known as garden mint, common mint, lamb mint and mackerel mint, is a species of mint, ''Mentha spicata'' (, native to Europe and southern temperate Asia, extending from Ireland in the west to southern China in the east. It is nat ...
whereas ''S''-(+)-carvone smells like caraway
Caraway, also known as meridian fennel and Persian cumin (''Carum carvi''), is a biennial plant in the family Apiaceae, native to western Asia, Europe, and North Africa.
Etymology
The etymology of "caraway" is unclear. Caraway has been ...
.
* Drug effectiveness: the antidepressant drug Citalopram
Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and so ...
is sold as a racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
mixture. However, studies have shown that only the (''S'')-(+) enantiomer is responsible for the drug's beneficial effects.
* Drug safety: D‑penicillamine is used in chelation therapy and for the treatment of rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are invol ...
whereas L‑penicillamine is toxic as it inhibits the action of pyridoxine, an essential B vitamin.
In biochemistry
Many biologically active molecules are chiral, including the naturally occurringamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s (the building blocks of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s) and sugars.
The origin of this homochirality
Homochirality is a uniformity of chirality, or handedness. Objects are chiral when they cannot be superposed on their mirror images. For example, the left and right hands of a human are approximately mirror images of each other but are not their ow ...
in biology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary i ...
is the subject of much debate. Most scientists believe that Earth life's "choice" of chirality was purely random, and that if carbon-based life forms exist elsewhere in the universe, their chemistry could theoretically have opposite chirality. However, there is some suggestion that early amino acids could have formed in comet dust. In this case, circularly polarised radiation (which makes up 17% of stellar radiation) could have caused the selective destruction of one chirality of amino acids, leading to a selection bias which ultimately resulted in all life on Earth being homochiral.
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s, which are chiral, often distinguish between the two enantiomers of a chiral substrate. One could imagine an enzyme as having a glove-like cavity that binds a substrate. If this glove is right-handed, then one enantiomer will fit inside and be bound, whereas the other enantiomer will have a poor fit and is unlikely to bind.
-forms of amino acids tend to be tasteless, whereas -forms tend to taste sweet. Spearmint
Spearmint, also known as garden mint, common mint, lamb mint and mackerel mint, is a species of mint, ''Mentha spicata'' (, native to Europe and southern temperate Asia, extending from Ireland in the west to southern China in the east. It is nat ...
leaves contain the -enantiomer of the chemical carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill.
Uses
Both c ...
or ''R''-(−)-carvone and caraway
Caraway, also known as meridian fennel and Persian cumin (''Carum carvi''), is a biennial plant in the family Apiaceae, native to western Asia, Europe, and North Africa.
Etymology
The etymology of "caraway" is unclear. Caraway has been ...
seeds contain the -enantiomer or ''S''-(+)-carvone. The two smell different to most people because our olfactory receptors are chiral.
Chirality is important in context of ordered phases as well, for example the addition of a small amount of an optically active molecule to a nematic phase (a phase that has long range orientational order of molecules) transforms that phase to a chiral nematic phase (or cholesteric phase). Chirality in context of such phases in polymeric fluids has also been studied in this context.
In inorganic chemistry
 Chirality is a symmetry property, not a property of any part of the periodic table. Thus many inorganic materials, molecules, and ions are chiral.
Chirality is a symmetry property, not a property of any part of the periodic table. Thus many inorganic materials, molecules, and ions are chiral. Quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
is an example from the mineral kingdom. Such noncentric materials are of interest for applications in nonlinear optics
Nonlinear optics (NLO) is the branch of optics that describes the behaviour of light in ''nonlinear media'', that is, media in which the polarization density P responds non-linearly to the electric field E of the light. The non-linearity is typic ...
.
In the areas of coordination chemistry and organometallic chemistry, chirality is pervasive and of practical importance. A famous example is tris(bipyridine)ruthenium(II) complex in which the three bipyridine ligands adopt a chiral propeller-like arrangement. The two enantiomers of complexes such as u(2,2′-bipyridine)3sup>2+ may be designated as Λ (capital lambda
Lambda (}, ''lám(b)da'') is the 11th letter of the Greek alphabet, representing the voiced alveolar lateral approximant . In the system of Greek numerals, lambda has a value of 30. Lambda is derived from the Phoenician Lamed . Lambda gave ri ...
, the Greek version of "L") for a left-handed twist of the propeller described by the ligands, and Δ (capital delta, Greek "D") for a right-handed twist (pictured). Also cf. dextro- and levo- (laevo-).
Chiral ligands confer chirality to a metal complex, as illustrated by metal-amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
complexes. If the metal exhibits catalytic properties, its combination with a chiral ligand is the basis of asymmetric catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
.
Methods and practices
The term '' optical activity'' is derived from the interaction of chiral materials with polarized light. In a solution, the (−)-form, or levorotatory form, of an optical isomer rotates the plane of a beam of linearly polarized lightcounterclockwise
Two-dimensional rotation can occur in two possible directions. Clockwise motion (abbreviated CW) proceeds in the same direction as a clock's hands: from the top to the right, then down and then to the left, and back up to the top. The opposite ...
. The (+)-form, or dextrorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
form, of an optical isomer does the opposite. The rotation of light is measured using a polarimeter and is expressed as the optical rotation.
Enantiomers can be separated by chiral resolution
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term wi ...
. This often involves forming crystals of a salt composed of one of the enantiomers and an acid or base from the so-called chiral pool The chiral pool is a "collection of abundant enantiopure building blocks provided by nature" used in synthesis. In other words, a chiral pool would be a large quantity of common organic enantiomers. Contributors to the chiral pool are amino acids, s ...
of naturally occurring chiral compounds, such as malic acid
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
or the amine brucine. Some racemic mixtures spontaneously crystallize into right-handed and left-handed crystals that can be separated by hand. Louis Pasteur used this method to separate left-handed and right-handed sodium ammonium tartrate crystals in 1849. Sometimes it is possible to seed a racemic solution with a right-handed and a left-handed crystal so that each will grow into a large crystal.
Liquid chromatography (HPLC and TLC) may also used as an analytical method for the direct separation of enantiomers and the control of enantiomeric purity, e.g. active pharmaceutical ingredients (API
An application programming interface (API) is a way for two or more computer programs to communicate with each other. It is a type of software interface, offering a service to other pieces of software. A document or standard that describes how ...
s) which are chiral.
Miscellaneous nomenclature
* Any non-racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
chiral substance is called scalemic. Scalemic materials can be enantiopure or enantioenriched.
* A chiral substance is enantiopure when only one of two possible enantiomers is present so that all molecules within a sample have the same chirality sense. Use of homochiral as a synonym is strongly discouraged.
* A chiral substance is enantioenriched or heterochiral when its enantiomeric ratio is greater than 50:50 but less than 100:0.
* Enantiomeric excess or e.e. is the difference between how much of one enantiomer is present compared to the other. For example, a sample with 40% e.e. of ''R'' contains 70% ''R'' and 30% ''S'' (70% − 30% = 40%).
History
The rotation of plane polarized light by chiral substances was first observed byJean-Baptiste Biot
Jean-Baptiste Biot (; ; 21 April 1774 – 3 February 1862) was a French physicist, astronomer, and mathematician who co-discovered the Biot–Savart law of magnetostatics with Félix Savart, established the reality of meteorites, made an early ba ...
in 1812, and gained considerable importance in the sugar industry, analytical chemistry, and pharmaceuticals. Louis Pasteur deduced in 1848 that this phenomenon has a molecular basis. The term ''chirality'' itself was coined by Lord Kelvin
William Thomson, 1st Baron Kelvin, (26 June 182417 December 1907) was a British mathematician, mathematical physicist and engineer born in Belfast. Professor of Natural Philosophy at the University of Glasgow for 53 years, he did important ...
in 1894. Different enantiomers or diastereomers of a compound were formerly called optical isomers due to their different optical properties. At one time, chirality was thought to be restricted to organic chemistry, but this misconception was overthrown by the resolution of a purely inorganic compound, a cobalt complex called hexol, by Alfred Werner in 1911.
In the early 1970s, various groups established that the human olfactory organ is capable of distinguishing chiral compounds.
See also
* Chirality (electromagnetism) *Chirality (mathematics)
In geometry, a figure is chiral (and said to have chirality) if it is not identical to its mirror image, or, more precisely, if it cannot be mapped to its mirror image by rotations and translations alone. An object that is not chiral is said to be ...
* Chirality (physics)
A chiral phenomenon is one that is not identical to its mirror image (see the article on mathematical chirality). The spin of a particle may be used to define a handedness, or helicity, for that particle, which, in the case of a massless particle ...
* Enantiopure drug An enantiopure drug is a pharmaceutical that is available in one specific enantiomeric form. Most biological molecules (proteins, sugars, etc.) are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind d ...
* Enantioselective synthesis
* Handedness
* Orientation (vector space)
The orientation of a real vector space or simply orientation of a vector space is the arbitrary choice of which ordered bases are "positively" oriented and which are "negatively" oriented. In the three-dimensional Euclidean space, right-handed ...
* Pfeiffer effect
* Stereochemistry for overview of stereochemistry in general
* Stereoisomerism
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
* Supramolecular chirality
References
Further reading
* * * *External links
21st International Symposium on Chirality
* ttps://web.archive.org/web/20071226005203/http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA3t5.html IUPAC nomenclature for amino acid configurations.
Michigan State University's explanation of R/S nomenclature
Chirality and the Search for Extraterrestrial Life
* The Handedness of the Universe by Roger A Hegstrom and Dilip K Kondepudi http://quantummechanics.ucsd.edu/ph87/ScientificAmerican/Sciam/Hegstrom_The_Handedness_of_the_universe.pdf {{DEFAULTSORT:Chirality (Chemistry) Stereochemistry Polarization (waves) Chirality Chemical nomenclature Biochemistry Origin of life Pharmacology