Caustic soda on:
[Wikipedia]
[Google]
[Amazon]
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive, caustic base (chemistry), base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound.
As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
Sodium hydroxide is used in many industries: in the manufacture of wood pulp, pulp and paper, textiles, drinking water, soaps and detergents, and as a drain cleaner. Worldwide production in 2004 was approximately 60 million tons, while demand was 51 million tons.
Solid-Liquid Equilibrium (SLE) and Vapour-Liquid Equilibrium (VLE) of Aqueous NaOH
. Online report, accessed on 2017-04-29. When solutions with less than 18.4% NaOH are cooled, water ice crystallizes first, leaving the NaOH in solution. The α form of the tetrahydrate has density 1.33 g/cm3. It melts congruously at 7.55 °C into a liquid with 35.7% NaOH and density 1.392 g/cm3, and therefore floats on it like ice on water. However, at about 4.9 °C it may instead melt incongruously into a mixture of solid and a liquid solution. The β form of the tetrahydrate is metastable, and often transforms spontaneously to the α form when cooled below −20 °C. Once initiated, the exothermic transformation is complete in a few minutes, with a 6.5% increase in volume of the solid. The β form can be crystallized from supercooled solutions at −26 °C, and melts partially at −1.83 °C. The "sodium hydroxide" of commerce is often the monohydrate (density 1.829 g/cm3). Physical data in technical literature may refer to this form, rather than the anhydrous compound.
5th edition, John Wiley & Sons. Historically, sodium hydroxide was produced by treating sodium carbonate with calcium hydroxide in a Salt metathesis reaction, metathesis reaction which takes advantage of the fact that sodium hydroxide is soluble, while calcium carbonate is not. This process was called causticizing. : This process was superseded by the Solvay process in the late 19th century, which was in turn supplanted by the Leblanc process and then chloralkali process which is in use today. Sodium hydroxide is also produced by combining pure sodium metal with water. The byproducts are hydrogen gas and heat, often resulting in a flame. : This reaction is commonly used for demonstrating the reactivity of alkali metals in academic environments; however, it is not commercially viable, as the isolation of sodium metal is typically performed by reduction or electrolysis of sodium compounds including sodium hydroxide.
Clean green finish that sends a loved one down the drain
Times Online. Retrieved 2013-02-20.Thacker, H. Leon; Kastner, Justin (August 2004)
''Carcass Disposal: A Comprehensive Review. Chapter 6''
National Agricultural Biosecurity Center, Kansas State University, 2004. Retrieved 2010-03-08 and the only solids that remain are bone hulls, which can be crushed between one's fingertips.Roach, Mary (2004). ''Stiff: The Curious Lives of Human Cadavers'', New York: W.W. Norton & Company. . Sodium hydroxide is frequently used in the process of decomposing roadkill dumped in landfills by animal disposal contractors. Due to its availability and low cost, it has been used by criminals to dispose of corpses. Italian serial killer Leonarda Cianciulli used this chemical to turn dead bodies into soap. In Mexico, a man who worked for drug cartels admitted disposing of over 300 bodies with it. Sodium hydroxide is a dangerous chemical due to its ability to hydrolyze protein. If a dilute solution is spilled on the skin, burns may result if the area is not washed thoroughly and for several minutes with running water. Splashes in the eye can be more serious and can lead to blindness.

 Sodium hydroxide is used in the home as a type of drain cleaner#Alkaline drain openers, drain opener to unblock clogged drains, usually in the form of a dry crystal or as a thick liquid gel. The alkali dissolves fat, greases to produce water soluble product (chemistry), products. It also hydrolysis, hydrolyzes proteins, such as those found in hair, which may block water pipes. These reactions are sped by the exothermic, heat generated when sodium hydroxide and the other chemical components of the cleaner dissolve in water. Such drain cleaner#Alkaline drain openers, alkaline drain cleaners and their drain cleaner#Acidic drain openers, acidic versions are highly corrosive and should be handled with great caution.
Sodium hydroxide is used in the home as a type of drain cleaner#Alkaline drain openers, drain opener to unblock clogged drains, usually in the form of a dry crystal or as a thick liquid gel. The alkali dissolves fat, greases to produce water soluble product (chemistry), products. It also hydrolysis, hydrolyzes proteins, such as those found in hair, which may block water pipes. These reactions are sped by the exothermic, heat generated when sodium hydroxide and the other chemical components of the cleaner dissolve in water. Such drain cleaner#Alkaline drain openers, alkaline drain cleaners and their drain cleaner#Acidic drain openers, acidic versions are highly corrosive and should be handled with great caution.
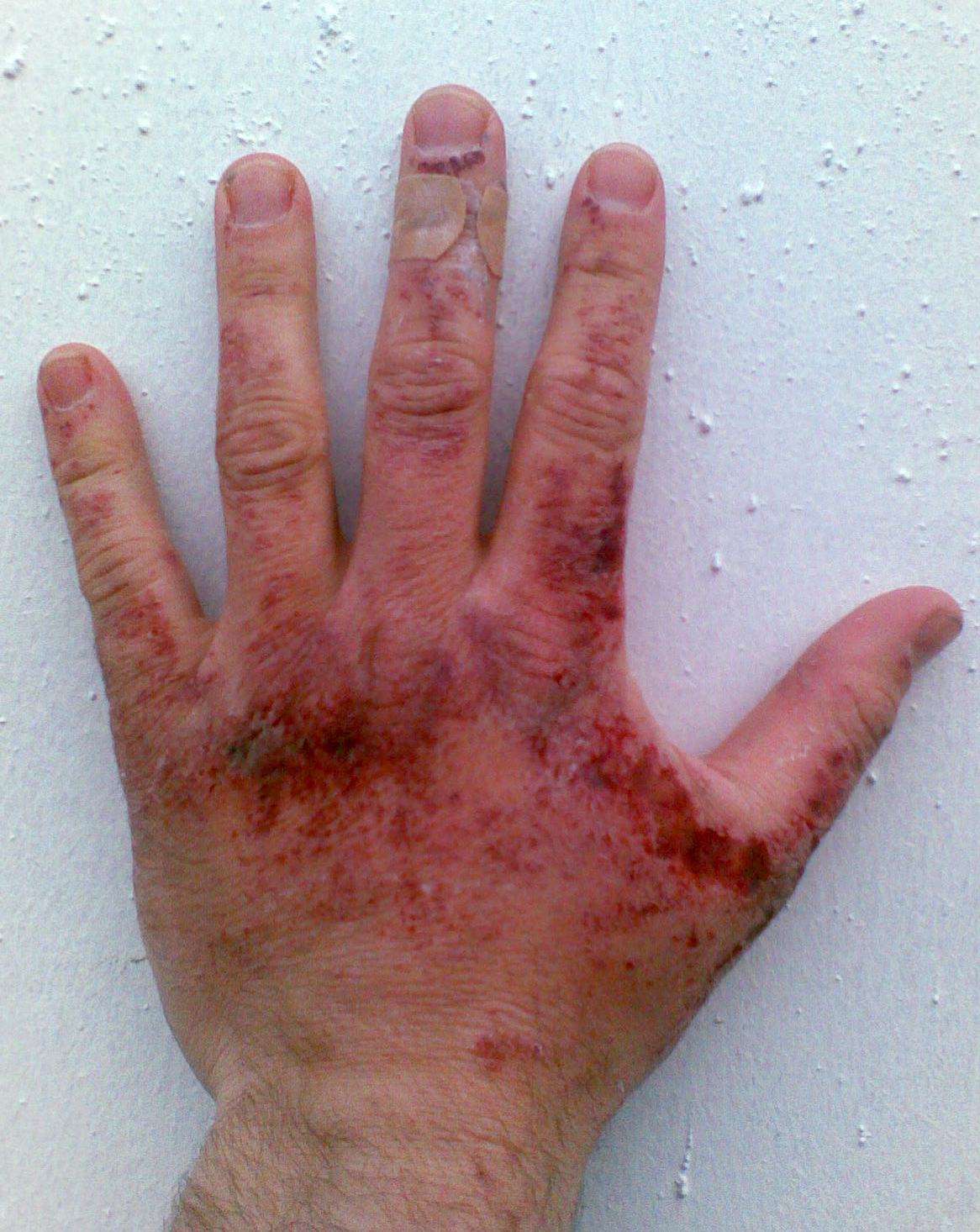 Like other corrosive acids and alkalis, drops of sodium hydroxide solutions can readily decompose proteins and lipids in Tissue (biology), living tissues via Hydrolysis#Esters and amides, amide hydrolysis and ester hydrolysis, which consequently cause chemical burns and may induce permanent blindness upon contact with eyes. Solid alkali can also express its corrosive nature if there is water, such as water vapor. Thus, protective equipment, like rubber gloves, safety clothing and eye protection, should always be used when handling this chemical or its solutions. The standard first aid measures for alkali spills on the skin is, as for other corrosives, irrigation with large quantities of water. Washing is continued for at least ten to fifteen minutes.
Moreover, solvation, dissolution of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables. It also produces heat when reacted with acids.
Sodium hydroxide is also mildly corrosive to glass, which can cause damage to glazing (window), glazing or cause ground glass joints to bind. Sodium hydroxide is corrosive to several metals, like aluminium which reacts with the alkali to produce flammable hydrogen gas on contact:
Like other corrosive acids and alkalis, drops of sodium hydroxide solutions can readily decompose proteins and lipids in Tissue (biology), living tissues via Hydrolysis#Esters and amides, amide hydrolysis and ester hydrolysis, which consequently cause chemical burns and may induce permanent blindness upon contact with eyes. Solid alkali can also express its corrosive nature if there is water, such as water vapor. Thus, protective equipment, like rubber gloves, safety clothing and eye protection, should always be used when handling this chemical or its solutions. The standard first aid measures for alkali spills on the skin is, as for other corrosives, irrigation with large quantities of water. Washing is continued for at least ten to fifteen minutes.
Moreover, solvation, dissolution of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables. It also produces heat when reacted with acids.
Sodium hydroxide is also mildly corrosive to glass, which can cause damage to glazing (window), glazing or cause ground glass joints to bind. Sodium hydroxide is corrosive to several metals, like aluminium which reacts with the alkali to produce flammable hydrogen gas on contact:
 Careful storage is needed when handling sodium hydroxide for use, especially bulk volumes. Following proper NaOH storage guidelines and maintaining worker/environment safety is always recommended given the chemical's burn hazard.
Sodium hydroxide is often stored in bottles for small-scale laboratory use, within intermediate bulk containers (medium volume containers) for cargo handling and transport, or within large stationary storage tanks with volumes up to 100,000 gallons for manufacturing or waste water plants with extensive NaOH use. Common materials that are compatible with sodium hydroxide and often utilized for NaOH storage include: polyethylene (HDPE, usual, Cross-linked polyethylene, XLPE, less common), carbon steel, polyvinyl chloride (PVC), stainless steel, and fiberglass reinforced plastic (FRP, with a resistant liner).
Sodium hydroxide must be stored in airtight containers to preserve its Equivalent concentration, normality as it will absorb water from the atmosphere.
Careful storage is needed when handling sodium hydroxide for use, especially bulk volumes. Following proper NaOH storage guidelines and maintaining worker/environment safety is always recommended given the chemical's burn hazard.
Sodium hydroxide is often stored in bottles for small-scale laboratory use, within intermediate bulk containers (medium volume containers) for cargo handling and transport, or within large stationary storage tanks with volumes up to 100,000 gallons for manufacturing or waste water plants with extensive NaOH use. Common materials that are compatible with sodium hydroxide and often utilized for NaOH storage include: polyethylene (HDPE, usual, Cross-linked polyethylene, XLPE, less common), carbon steel, polyvinyl chloride (PVC), stainless steel, and fiberglass reinforced plastic (FRP, with a resistant liner).
Sodium hydroxide must be stored in airtight containers to preserve its Equivalent concentration, normality as it will absorb water from the atmosphere.
/ref> A procedure for making sodium hydroxide appeared as part of a recipe for making soap in an Arab book of the late 13th century: (Inventions from the Various Industrial Arts), which was compiled by al-Muzaffar Yusuf ibn 'Umar ibn 'Ali ibn Rasul (d. 1295), a king of Yemen. The recipe called for passing water repeatedly through a mixture of ''alkali'' (Arabic: , where is ash from Glasswort, saltwort plants, which are rich in sodium; hence ''alkali'' was impure sodium carbonate) and quicklime (calcium oxide, CaO), whereby a solution of sodium hydroxide was obtained. European soap makers also followed this recipe. When in 1791 the French chemist and surgeon Nicolas Leblanc (1742–1806) patented a Leblanc process, process for mass-producing sodium carbonate, natural "soda ash" (impure sodium carbonate that was obtained from the ashes of plants that are rich in sodium) was replaced by this artificial version. However, by the 20th century, the Chloralkali process, electrolysis of sodium chloride had become the primary method for producing sodium hydroxide.O'Brien, Thomas F.; Bommaraju, Tilak V. and Hine, Fumio (2005) ''Handbook of Chlor-Alkali Technology'', vol. 1. Berlin, Germany: Springer. Chapter 2: History of the Chlor-Alkali Industry, p. 34.
International Chemical Safety Card 0360
*Euro Chlor-How is chlorine made
Chlorine OnlineCDC – Sodium Hydroxide – NIOSH Workplace Safety and Health Topic
*Data sheets
Technical charts (page 33—41)
for enthalpy, temperature and pressure
Sodium Hydroxide MSDS
Certified Lye MSDS
Hill Brothers MSDS
*[https://web.archive.org/web/20150508191719/http://www.inclusive-science-engineering.com/inorganic-chemical-caustic-soda-production-process-description-and-flowsheet/ Caustic soda production in continuous causticising plant by lime soda process] {{DEFAULTSORT:Sodium Hydroxide Chemical engineering Cleaning products Deliquescent substances Desiccants Household chemicals Hydroxides Inorganic compounds Photographic chemicals Sodium compounds E-number additives Food acidity regulators
Properties
Physical properties
Pure sodium hydroxide is a colorless crystalline solid that melts at without decomposition, and with a boiling point of . It is highly soluble in water, with a lower solubility in Chemical polarity, polar Solvent, solvents such as ethanol and methanol. NaOH is insoluble in Ether, ether and other non-polar solvents. Similar to the hydration of sulfuric acid, dissolution (chemistry), dissolution of solid sodium hydroxide in water is a highly exothermic reaction where a large amount of heat is liberated, posing a threat to safety through the possibility of splashing. The resulting solution is usually colorless and odorless. As with other alkaline solutions, it feels slippery with skin contact due to the process of saponification that occurs between NaOH and natural skin oils.Viscosity
Concentrated (50%) aqueous solutions of sodium hydroxide have a characteristic viscosity, 78 mPascal (unit), Pa·s, that is much greater than that of water (1.0 mPa·s) and near that of olive oil (85 mPa·s) at room temperature. The viscosity of aqueous NaOH, as with any liquid chemical, is inversely related to its service temperature, i.e., its viscosity decreases as temperature increases, and vice versa. The viscosity of sodium hydroxide solutions plays a direct role in its application as well as its storage.Hydrates
Sodium hydroxide can form several hydrates , which result in a complex solubility diagram that was described in detail by Spencer Umfreville Pickering, S. U. Pickering in 1893. The known hydrates and the approximate ranges of temperature and concentration (mass percent of NaOH) of their Saturated solution, saturated water solutions are: *Heptahydrate, : from −28 °C (18.8%) to −24 °C (22.2%). *Pentahydrate, : from −24 °C (22.2%) to −17.7 (24.8%). *Tetrahydrate, , α form: from −17.7 (24.8%) to +5.4 °C (32.5%). *Tetrahydrate, , β form: metastable. *Trihemihydrate, : from +5.4 °C (32.5%) to +15.38 °C (38.8%) and then to +5.0 °C (45.7%). *Trihydrate, : metastable. *Dihydrate, : from +5.0 °C (45.7%) to +12.3 °C (51%). *Monohydrate, : from +12.3 °C (51%) to 65.10 °C (69%) then to 62.63 °C (73.1%). Early reports refer to hydrates with ''n'' = 0.5 or ''n'' = 2/3, but later careful investigations failed to confirm their existence. The only hydrates with stable melting points are (65.10 °C) and (15.38 °C). The other hydrates, except the metastable ones and (β) can be crystallized from solutions of the proper composition, as listed above. However, solutions of NaOH can be easily supercooled by many degrees, which allows the formation of hydrates (including the metastable ones) from solutions with different concentrations. For example, when a solution of NaOH and water with 1:2 mole ratio (52.6% NaOH by mass) is cooled, the monohydrate normally starts to crystallize (at about 22 °C) before the dihydrate. However, the solution can easily be supercooled down to −15 °C, at which point it may quickly crystallize as the dihydrate. When heated, the solid dihydrate might melt directly into a solution at 13.35 °C; however, once the temperature exceeds 12.58 °C. it often decomposes into solid monohydrate and a liquid solution. Even the ''n'' = 3.5 hydrate is difficult to crystallize, because the solution supercools so much that other hydrates become more stable. A hot water solution containing 73.1% (mass) of NaOH is a eutectic that solidifies at about 62.63 °C as an intimate mix of anhydrous and monohydrate crystals. A second stable eutectic composition is 45.4% (mass) of NaOH, that solidifies at about 4.9 °C into a mixture of crystals of the dihydrate and of the 3.5-hydrate. The third stable eutectic has 18.4% (mass) of NaOH. It solidifies at about −28.7 °C as a mixture of water ice and the heptahydrate .M. Conde Engineering:Solid-Liquid Equilibrium (SLE) and Vapour-Liquid Equilibrium (VLE) of Aqueous NaOH
. Online report, accessed on 2017-04-29. When solutions with less than 18.4% NaOH are cooled, water ice crystallizes first, leaving the NaOH in solution. The α form of the tetrahydrate has density 1.33 g/cm3. It melts congruously at 7.55 °C into a liquid with 35.7% NaOH and density 1.392 g/cm3, and therefore floats on it like ice on water. However, at about 4.9 °C it may instead melt incongruously into a mixture of solid and a liquid solution. The β form of the tetrahydrate is metastable, and often transforms spontaneously to the α form when cooled below −20 °C. Once initiated, the exothermic transformation is complete in a few minutes, with a 6.5% increase in volume of the solid. The β form can be crystallized from supercooled solutions at −26 °C, and melts partially at −1.83 °C. The "sodium hydroxide" of commerce is often the monohydrate (density 1.829 g/cm3). Physical data in technical literature may refer to this form, rather than the anhydrous compound.
Crystal structure
NaOH and its monohydrate form orthorhombic crystals with the space groups Cmcm (Pearson symbol, oS8) and Pbca (oP24), respectively. The monohydrate cell dimensions are a = 1.1825, b = 0.6213, c = 0.6069 nanometer, nm. The atoms are arranged in a hydrargillite-like layer structure, with each sodium atom surrounded by six oxygen atoms, three each from hydroxide ions and three from water molecules. The hydrogen atoms of the hydroxyls form strong bonds with oxygen atoms within each O layer. Adjacent O layers are held together by hydrogen bonds between water molecules.Chemical properties
Reaction with acids
Sodium hydroxide reacts with protic acids to produce water and the corresponding salts. For example, when sodium hydroxide reacts with hydrochloric acid, sodium chloride is formed: : In general, such neutralization (chemistry), neutralization reactions are represented by one simple net ionic equation: : This type of reaction with a strong acid releases heat, and hence is exothermic reaction, exothermic. Such acid–base reactions can also be used for titrations. However, sodium hydroxide is not used as a primary standard because it is hygroscopic and absorbs carbon dioxide from air.Reaction with acidic oxides
Sodium hydroxide also reacts with acid anhydride, acidic oxides, such as sulfur dioxide. Such reactions are often used to "Scrubber, scrub" harmful acidic gases (like and ) produced in the burning of coal and thus prevent their release into the atmosphere. For example, :Reaction with metals and oxides
Glass reacts slowly with aqueous sodium hydroxide solutions at ambient temperatures to form soluble silicates. Because of this, glass joints and stopcocks exposed to sodium hydroxide have a tendency to "freeze". Laboratory flask, Flasks and glass-lined chemical reactors are damaged by long exposure to hot sodium hydroxide, which also frosts the glass. Sodium hydroxide does not attack iron at room temperatures, since iron does not have amphoteric properties (i.e., it only dissolves in acid, not base). Nevertheless, at high temperatures (e.g. above 500 °C), iron can react endothermically with sodium hydroxide to form iron(III) oxide, sodium metal, and hydrogen gas. This is due to the lower enthalpy of formation of iron(III) oxide (−824.2 kJ/mol) compared to sodium hydroxide (-500 kJ/mol) and positive entropy change of reaction, which imply spontaneity at high temperatures (, ) and non-spontaneity at low temperatures (, ). Consider the following reaction between molten sodium hydroxide and finely divided iron filings: : A few transition metals, however, may react vigorously with sodium hydroxide under milder conditions. In 1986, an aluminium tank truck, road tanker in the UK was mistakenly used to transport 25% sodium hydroxide solution, causing pressurization of the contents and damage to the tanker. The pressurization was due to the hydrogen gas which is produced in the reaction between sodium hydroxide and aluminium: :Precipitant
Unlike sodium hydroxide, which is soluble, the hydroxides of most transition metals are insoluble, and therefore sodium hydroxide can be used to precipitate transition metal hydroxides. The following colours are observed: *Copper - blue *Iron(II) - green *Iron(III) - yellow / brown Zinc and lead salts dissolve in excess sodium hydroxide to give a clear solution of or . Aluminium hydroxide is used as a gelatinous Flocculation#Flocculants, flocculant to filter out particulate matter in water treatment. Aluminium hydroxide is prepared at the treatment plant from aluminium sulfate by reacting it with sodium hydroxide or bicarbonate. : :Saponification
Sodium hydroxide can be used for the base-driven hydrolysis of esters (as in saponification), amides and alkyl halides. However, the limited solubility of sodium hydroxide in organic solvents means that the more soluble potassium hydroxide (KOH) is often preferred. Touching a sodium hydroxide solution with bare hands, while not recommended, produces a slippery feeling. This happens because oils on the skin such as sebum are converted to soap. Despite solubility in propylene glycol it is unlikely to replace water in saponification due to propylene glycol's primary reaction with fat before reaction between sodium hydroxide and fat.Production
Sodium hydroxide is industrially produced as a 50% solution by variations of the electrolytic chloralkali process. Chlorine gas is also produced in this process. Solid sodium hydroxide is obtained from this solution by the evaporation of water. Solid sodium hydroxide is most commonly sold as flakes, prills, and cast blocks. In 2004, world production was estimated at 60 million dry tonnes of sodium hydroxide, and demand was estimated at 51 million tonnes. In 1998, total world production was around 45 million tonnes. North America and Asia each contributed around 14 million tonnes, while Europe produced around 10 million tonnes. In the United States, the major producer of sodium hydroxide is Olin, which has annual production around 5.7 million tonnes from sites at Freeport, Texas, Freeport, Texas, and Plaquemine, Louisiana, Plaquemine, Louisiana, St Gabriel, Louisiana, McIntosh, Alabama, Charleston, Tennessee, Niagara Falls, New York, and Becancour, Canada. Other major US producers include Oxychem, PPG Industries, Westlake, Shintek and Formosa Plastics Group, Formosa. All of these companies use the chloralkali process.''Kirk-Othmer Encyclopedia of Chemical Technology''5th edition, John Wiley & Sons. Historically, sodium hydroxide was produced by treating sodium carbonate with calcium hydroxide in a Salt metathesis reaction, metathesis reaction which takes advantage of the fact that sodium hydroxide is soluble, while calcium carbonate is not. This process was called causticizing. : This process was superseded by the Solvay process in the late 19th century, which was in turn supplanted by the Leblanc process and then chloralkali process which is in use today. Sodium hydroxide is also produced by combining pure sodium metal with water. The byproducts are hydrogen gas and heat, often resulting in a flame. : This reaction is commonly used for demonstrating the reactivity of alkali metals in academic environments; however, it is not commercially viable, as the isolation of sodium metal is typically performed by reduction or electrolysis of sodium compounds including sodium hydroxide.
Uses
Sodium hydroxide is a popular strong base (chemistry), base used in industry. Sodium hydroxide is used in the manufacture of sodium salts and detergents, pH regulation, and organic synthesis. In bulk, it is most often handled as an aqueous solution, since solutions are cheaper and easier to handle. Sodium hydroxide is used in many scenarios where it is desirable to increase the alkalinity of a mixture, or to neutralize acids. For example, in the petroleum industry, sodium hydroxide is used as an additive in drilling mud to increase alkalinity in bentonite mud systems, to increase the mud viscosity, and to neutralize any acid gas (such as hydrogen sulfide and carbon dioxide) which may be encountered in the geological formation as drilling progresses. Another use is in Salt spray testing where pH needs to be regulated. Sodium hydroxide is used with hydrochloric acid to balance pH. The resultant salt, NaCl, is the corrosive agent used in the standard neutral pH salt spray test. Poor quality crude oil can be treated with sodium hydroxide to remove sulfurous impurities in a process known as ''caustic washing''. As above, sodium hydroxide reacts with weak acids such as hydrogen sulfide and mercaptans to yield non-volatile sodium salts, which can be removed. The waste which is formed is toxic and difficult to deal with, and the process is banned in many countries because of this. In 2006, Trafigura used the process and then 2006 Côte d'Ivoire toxic waste dump, dumped the waste in Ivory Coast. Other common uses of sodium hydroxide include: *for making soaps and detergents. Sodium hydroxide is used for hard bar soap, while potassium hydroxide is used for liquid soaps. Sodium hydroxide is used more often than potassium hydroxide because it is cheaper and a smaller quantity is needed. *as drain cleaners that contain sodium hydroxide convert fats and grease that can clog pipes into soap, which dissolves in water ''(see Sodium hydroxide#Cleaning agent, cleaning agent)''. *for making artificial textile fibres (such as Rayon). *in the manufacture of paper. Around 56% of sodium hydroxide produced is used by industry, 25% of which is used in the paper industry ''(see Sodium hydroxide#Chemical pulping, chemical pulping)''. *in purifying Bauxite, bauxite ore from which Aluminium, aluminium metal is extracted. This is known as Bayer process ''(see Sodium hydroxide#Dissolving amphoteric metals and compounds, dissolving amphoteric metals and compounds)''. *in de-greasing metals, oil refining, and making dyes and bleaches. *in water treatment plants for pH regulation. *to treat bagels and pretzel dough, giving the distinctive shiny finish.Chemical pulping
Sodium hydroxide is also widely used in pulping of wood for making paper or regenerated fibers. Along with sodium sulfide, sodium hydroxide is a key component of the white liquor solution used to separate lignin from cellulose fibers in the kraft process. It also plays a key role in several later stages of the process of Bleaching of wood pulp, bleaching the brown pulp resulting from the pulping process. These stages include oxygen delignification, oxidation, oxidative extraction, and simple extraction, all of which require a strong alkaline environment with a pH > 10.5 at the end of the stages.Tissue digestion
In a similar fashion, sodium hydroxide is used to digest tissues, as in a process that was used with farm animals at one time. This process involved placing a carcass into a sealed chamber, then adding a mixture of sodium hydroxide and water (which breaks the chemical bonds that keep the flesh intact). This eventually turns the body into a liquid with a dark brown color,Ayres, Chris (27 February 2010Clean green finish that sends a loved one down the drain
Times Online. Retrieved 2013-02-20.Thacker, H. Leon; Kastner, Justin (August 2004)
''Carcass Disposal: A Comprehensive Review. Chapter 6''
National Agricultural Biosecurity Center, Kansas State University, 2004. Retrieved 2010-03-08 and the only solids that remain are bone hulls, which can be crushed between one's fingertips.Roach, Mary (2004). ''Stiff: The Curious Lives of Human Cadavers'', New York: W.W. Norton & Company. . Sodium hydroxide is frequently used in the process of decomposing roadkill dumped in landfills by animal disposal contractors. Due to its availability and low cost, it has been used by criminals to dispose of corpses. Italian serial killer Leonarda Cianciulli used this chemical to turn dead bodies into soap. In Mexico, a man who worked for drug cartels admitted disposing of over 300 bodies with it. Sodium hydroxide is a dangerous chemical due to its ability to hydrolyze protein. If a dilute solution is spilled on the skin, burns may result if the area is not washed thoroughly and for several minutes with running water. Splashes in the eye can be more serious and can lead to blindness.
Dissolving amphoteric metals and compounds
Strong bases attack aluminium. Sodium hydroxide reacts with aluminium and water to release hydrogen gas. The aluminium takes the oxygen atom from sodium hydroxide, which in turn takes the oxygen atom from the water, and releases the two hydrogen atoms. The reaction thus produces hydrogen gas and sodium aluminate. In this reaction, sodium hydroxide acts as an agent to make the solution alkaline, which aluminium can dissolve in. : Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as , , , or . Formation of sodium tetrahydroxoaluminate(III) or hydrated sodium aluminate is given by: : This reaction can be useful in etching, removing anodizing, or converting a polished surface to a satin-like finish, but without further Passivation (chemistry), passivation such as anodizing or alodining the surface may become degraded, either under normal use or in severe atmospheric conditions. In the Bayer process, sodium hydroxide is used in the refining of alumina containing ores (bauxite) to produce alumina (aluminium oxide) which is the raw material used to produce aluminium metal via the electrolysis, electrolytic Hall-Héroult process. Since the alumina is amphoteric, it dissolves in the sodium hydroxide, leaving impurities less soluble at high pH such as iron oxides behind in the form of a highly alkaline red mud. Other amphoteric metals are zinc and lead which dissolve in concentrated sodium hydroxide solutions to give sodium zincate and sodium plumbate respectively.Esterification and transesterification reagent
Sodium hydroxide is traditionally used in soap making (cold process soap, saponification). It was made in the nineteenth century for a hard surface rather than liquid product because it was easier to store and transport. For the manufacture of biodiesel, sodium hydroxide is used as a catalyst for the transesterification of methanol and triglycerides. This only works with anhydrous sodium hydroxide, because combined with water the fat would turn into soap, which would be tainted with methanol. NaOH is used more often than potassium hydroxide because it is cheaper and a smaller quantity is needed. Due to production costs, NaOH, which is produced using common salt is cheaper than potassium hydroxide.Food preparation
Food uses of sodium hydroxide include washing or chemical peeling of fruits and vegetables, chocolate and Cocoa mass, cocoa processing, caramel coloring production, poultry scalding, soft drink processing, and thickening ice cream. Olives are often soaked in sodium hydroxide for softening; Pretzels and German lye rolls are glazed with a sodium hydroxide solution before baking to make them crisp. Owing to the difficulty in obtaining food grade sodium hydroxide in small quantities for home use, sodium carbonate is often used in place of sodium hydroxide. It is known as E number E524. Specific foods processed with sodium hydroxide include: *German pretzels are poached in a boiling sodium carbonate solution or cold sodium hydroxide solution before baking, which contributes to their unique crust. *Lye-water is an essential ingredient in the crust of the traditional baked Chinese moon cakes. *Most yellow coloured Chinese noodles are made with lye-water but are commonly mistaken for containing egg. *One variety of zongzi uses lye water to impart a sweet flavor. *Sodium hydroxide is also the chemical that causes gelling of egg whites in the production of Century eggs. *Some methods of preparing olives involve subjecting them to a lye-based brine. *The Filipino dessert ( fil, kakanin) called uses a small quantity of lye water to help give the rice flour batter a jelly like consistency. A similar process is also used in the kakanin known as or except that the mixture uses grated cassava instead of rice flour. *The Norway, Norwegian dish known as lutefisk ( no, lutfisk, lit=lye fish). *Bagels are often boiled in a lye solution before baking, contributing to their shiny crust. *Hominy is dried maize (corn) kernels reconstituted by soaking in lye-water. These expand considerably in size and may be further processed by frying to make corn nuts or by drying and grinding to make grits. Hominy is used to create Masa, a popular flour used in Mexican cuisine to make Corn tortillas and tamales. Nixtamal is similar, but uses calcium hydroxide instead of sodium hydroxide.Cleaning agent
Sodium hydroxide is frequently used as an industrial cleaning agent where it is often called "caustic". It is added to water, heated, and then used to clean process equipment, storage tanks, etc. It can dissolve grease (lubricant), grease, oils, fats and protein-based deposits. It is also used for cleaning waste discharge pipes under sinks and drains in domestic properties. Surfactants can be added to the sodium hydroxide solution in order to stabilize dissolved substances and thus prevent redeposition. A sodium hydroxide soak solution is used as a powerful degreaser on stainless steel and glass bakeware. It is also a common ingredient in oven cleaners. A common use of sodium hydroxide is in the production of parts washer detergents. Parts washer detergents based on sodium hydroxide are some of the most aggressive parts washer cleaning chemicals. The sodium hydroxide-based detergents include surfactants, rust inhibitors and defoamers. A parts washer heats water and the detergent in a closed cabinet and then sprays the heated sodium hydroxide and hot water at pressure against dirty parts for degreasing applications. Sodium hydroxide used in this manner replaced many solvent-based systems in the early 1990s when 1,1,1-Trichloroethane, trichloroethane was outlawed by the Montreal Protocol. Water and sodium hydroxide detergent-based parts washers are considered to be an environmental improvement over the solvent-based cleaning methods.
 Sodium hydroxide is used in the home as a type of drain cleaner#Alkaline drain openers, drain opener to unblock clogged drains, usually in the form of a dry crystal or as a thick liquid gel. The alkali dissolves fat, greases to produce water soluble product (chemistry), products. It also hydrolysis, hydrolyzes proteins, such as those found in hair, which may block water pipes. These reactions are sped by the exothermic, heat generated when sodium hydroxide and the other chemical components of the cleaner dissolve in water. Such drain cleaner#Alkaline drain openers, alkaline drain cleaners and their drain cleaner#Acidic drain openers, acidic versions are highly corrosive and should be handled with great caution.
Sodium hydroxide is used in the home as a type of drain cleaner#Alkaline drain openers, drain opener to unblock clogged drains, usually in the form of a dry crystal or as a thick liquid gel. The alkali dissolves fat, greases to produce water soluble product (chemistry), products. It also hydrolysis, hydrolyzes proteins, such as those found in hair, which may block water pipes. These reactions are sped by the exothermic, heat generated when sodium hydroxide and the other chemical components of the cleaner dissolve in water. Such drain cleaner#Alkaline drain openers, alkaline drain cleaners and their drain cleaner#Acidic drain openers, acidic versions are highly corrosive and should be handled with great caution.
Relaxer
Sodium hydroxide is used in some relaxers to straighten hair. However, because of the high incidence and intensity of chemical burns, manufacturers of chemical relaxers use other alkaline chemicals in preparations available to consumers. Sodium hydroxide relaxers are still available, but they are used mostly by professionals.Paint stripper
A solution of sodium hydroxide in water was traditionally used as the most common paint stripper on wooden objects. Its use has become less common, because it can damage the wood surface, raising the grain and staining the colour.Water treatment
Sodium hydroxide is sometimes used during water purification to raise the pH of water supplies. Increased pH makes the water less corrosive to plumbing and reduces the amount of lead, copper and other toxic metals that can dissolve into drinking water.Historical uses
Sodium hydroxide has been used for detection of carbon monoxide poisoning, with blood samples of such patients turning to a vermilion color upon the addition of a few drops of sodium hydroxide. Today, carbon monoxide poisoning can be detected by CO oximetry.In cement mixes, mortars, concrete, grouts
Sodium hydroxide is used in some cement mix plasticisers. This helps homogenise cement mixes, preventing segregation of sands and cement, decreases the amount of water required in a mix and increases workability of the cement product, be it mortar, render or concrete.Experimental
Flavonoids
See: Sodium hydroxide test for flavonoidsSummer-winter heat storage
After decades of research, EMPA researchers and others are experimenting with concentrated sodium hydroxide (NaOH) as the thermal storage or seasonal reservoir medium for power plants and domestic HVAC, space-heating. If water is added to solid or concentrated sodium hydroxide (NaOH), heat is released. The dilution is Exothermic process, exothermic – chemical energy is released in the form of heat. Conversely, by applying heat energy into a dilute sodium hydroxide solution the water will evaporate so that the solution becomes more concentrated and thus stores the supplied heat as Endothermic process, latent chemical energy.Neutron moderator
Seaborg Technologies is working on a reactor design in which NaOH is used as a neutron moderator,Safety
 Like other corrosive acids and alkalis, drops of sodium hydroxide solutions can readily decompose proteins and lipids in Tissue (biology), living tissues via Hydrolysis#Esters and amides, amide hydrolysis and ester hydrolysis, which consequently cause chemical burns and may induce permanent blindness upon contact with eyes. Solid alkali can also express its corrosive nature if there is water, such as water vapor. Thus, protective equipment, like rubber gloves, safety clothing and eye protection, should always be used when handling this chemical or its solutions. The standard first aid measures for alkali spills on the skin is, as for other corrosives, irrigation with large quantities of water. Washing is continued for at least ten to fifteen minutes.
Moreover, solvation, dissolution of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables. It also produces heat when reacted with acids.
Sodium hydroxide is also mildly corrosive to glass, which can cause damage to glazing (window), glazing or cause ground glass joints to bind. Sodium hydroxide is corrosive to several metals, like aluminium which reacts with the alkali to produce flammable hydrogen gas on contact:
Like other corrosive acids and alkalis, drops of sodium hydroxide solutions can readily decompose proteins and lipids in Tissue (biology), living tissues via Hydrolysis#Esters and amides, amide hydrolysis and ester hydrolysis, which consequently cause chemical burns and may induce permanent blindness upon contact with eyes. Solid alkali can also express its corrosive nature if there is water, such as water vapor. Thus, protective equipment, like rubber gloves, safety clothing and eye protection, should always be used when handling this chemical or its solutions. The standard first aid measures for alkali spills on the skin is, as for other corrosives, irrigation with large quantities of water. Washing is continued for at least ten to fifteen minutes.
Moreover, solvation, dissolution of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables. It also produces heat when reacted with acids.
Sodium hydroxide is also mildly corrosive to glass, which can cause damage to glazing (window), glazing or cause ground glass joints to bind. Sodium hydroxide is corrosive to several metals, like aluminium which reacts with the alkali to produce flammable hydrogen gas on contact:
Storage
 Careful storage is needed when handling sodium hydroxide for use, especially bulk volumes. Following proper NaOH storage guidelines and maintaining worker/environment safety is always recommended given the chemical's burn hazard.
Sodium hydroxide is often stored in bottles for small-scale laboratory use, within intermediate bulk containers (medium volume containers) for cargo handling and transport, or within large stationary storage tanks with volumes up to 100,000 gallons for manufacturing or waste water plants with extensive NaOH use. Common materials that are compatible with sodium hydroxide and often utilized for NaOH storage include: polyethylene (HDPE, usual, Cross-linked polyethylene, XLPE, less common), carbon steel, polyvinyl chloride (PVC), stainless steel, and fiberglass reinforced plastic (FRP, with a resistant liner).
Sodium hydroxide must be stored in airtight containers to preserve its Equivalent concentration, normality as it will absorb water from the atmosphere.
Careful storage is needed when handling sodium hydroxide for use, especially bulk volumes. Following proper NaOH storage guidelines and maintaining worker/environment safety is always recommended given the chemical's burn hazard.
Sodium hydroxide is often stored in bottles for small-scale laboratory use, within intermediate bulk containers (medium volume containers) for cargo handling and transport, or within large stationary storage tanks with volumes up to 100,000 gallons for manufacturing or waste water plants with extensive NaOH use. Common materials that are compatible with sodium hydroxide and often utilized for NaOH storage include: polyethylene (HDPE, usual, Cross-linked polyethylene, XLPE, less common), carbon steel, polyvinyl chloride (PVC), stainless steel, and fiberglass reinforced plastic (FRP, with a resistant liner).
Sodium hydroxide must be stored in airtight containers to preserve its Equivalent concentration, normality as it will absorb water from the atmosphere.
History
Sodium hydroxide was first prepared by soap makers.Thorpe, Thomas Edward, ed., ''A Dictionary of Applied Chemistry'' (London, England: Longmans, Green, and Co., 1913), vol. 5/ref> A procedure for making sodium hydroxide appeared as part of a recipe for making soap in an Arab book of the late 13th century: (Inventions from the Various Industrial Arts), which was compiled by al-Muzaffar Yusuf ibn 'Umar ibn 'Ali ibn Rasul (d. 1295), a king of Yemen. The recipe called for passing water repeatedly through a mixture of ''alkali'' (Arabic: , where is ash from Glasswort, saltwort plants, which are rich in sodium; hence ''alkali'' was impure sodium carbonate) and quicklime (calcium oxide, CaO), whereby a solution of sodium hydroxide was obtained. European soap makers also followed this recipe. When in 1791 the French chemist and surgeon Nicolas Leblanc (1742–1806) patented a Leblanc process, process for mass-producing sodium carbonate, natural "soda ash" (impure sodium carbonate that was obtained from the ashes of plants that are rich in sodium) was replaced by this artificial version. However, by the 20th century, the Chloralkali process, electrolysis of sodium chloride had become the primary method for producing sodium hydroxide.O'Brien, Thomas F.; Bommaraju, Tilak V. and Hine, Fumio (2005) ''Handbook of Chlor-Alkali Technology'', vol. 1. Berlin, Germany: Springer. Chapter 2: History of the Chlor-Alkali Industry, p. 34.
See also
*pH, Acid and base *HAZMAT Class 8 Corrosive Substances *List of cleaning agentsReferences
Bibliography
*External links
International Chemical Safety Card 0360
*Euro Chlor-How is chlorine made
Chlorine Online
*Data sheets
Technical charts (page 33—41)
for enthalpy, temperature and pressure
Sodium Hydroxide MSDS
Certified Lye MSDS
Hill Brothers MSDS
*[https://web.archive.org/web/20150508191719/http://www.inclusive-science-engineering.com/inorganic-chemical-caustic-soda-production-process-description-and-flowsheet/ Caustic soda production in continuous causticising plant by lime soda process] {{DEFAULTSORT:Sodium Hydroxide Chemical engineering Cleaning products Deliquescent substances Desiccants Household chemicals Hydroxides Inorganic compounds Photographic chemicals Sodium compounds E-number additives Food acidity regulators