Carbanion on:
[Wikipedia]
[Google]
[Amazon]
In R3CH\, + \ddot^- -> \mathbf + HB
where B stands for the base. The carbanions formed from

 However, ''delocalized'' carbanions may deviate from these geometries. Instead of residing in a hybrid orbital, the carbanionic lone pair may instead occupy a p orbital (or an orbital of high p character). A p orbital has a more suitable shape and orientation to overlap with the neighboring π system, resulting in more effective charge delocalization. As a consequence, alkyl carbanions with neighboring conjugating groups (e.g., allylic anions, enolates, nitronates, etc.) are generally planar rather than pyramidized. Likewise, delocalized alkenyl carbanions sometimes favor a linear instead of bent geometry. More often, a bent geometry is still preferred for substituted alkenyl anions, though the linear geometry is only ''slightly'' less stable, resulting in facile equilibration between the (''E'') and (''Z'') isomers of the (bent) anion through a linear transition state. For instance, calculations indicate that the parent vinyl anion, H2C=CH−, has an inversion barrier of , while allenyl anion, H2C=C=H (↔ H2–C≡CH), whose negative charge is stabilized by delocalization, has an inversion barrier of only , reflecting stabilization of the linear transition state by better π delocalization.
However, ''delocalized'' carbanions may deviate from these geometries. Instead of residing in a hybrid orbital, the carbanionic lone pair may instead occupy a p orbital (or an orbital of high p character). A p orbital has a more suitable shape and orientation to overlap with the neighboring π system, resulting in more effective charge delocalization. As a consequence, alkyl carbanions with neighboring conjugating groups (e.g., allylic anions, enolates, nitronates, etc.) are generally planar rather than pyramidized. Likewise, delocalized alkenyl carbanions sometimes favor a linear instead of bent geometry. More often, a bent geometry is still preferred for substituted alkenyl anions, though the linear geometry is only ''slightly'' less stable, resulting in facile equilibration between the (''E'') and (''Z'') isomers of the (bent) anion through a linear transition state. For instance, calculations indicate that the parent vinyl anion, H2C=CH−, has an inversion barrier of , while allenyl anion, H2C=C=H (↔ H2–C≡CH), whose negative charge is stabilized by delocalization, has an inversion barrier of only , reflecting stabilization of the linear transition state by better π delocalization.
 Adding ''n''-butyllithium to
Adding ''n''-butyllithium to
 On heating the reaction to 0 °C the optical activity is lost. More evidence followed in the 1960s. A reaction of the ''cis'' isomer of 2-methylcyclopropyl bromide with ''s''-butyllithium again followed by
On heating the reaction to 0 °C the optical activity is lost. More evidence followed in the 1960s. A reaction of the ''cis'' isomer of 2-methylcyclopropyl bromide with ''s''-butyllithium again followed by  In the same manner the reaction of (+)-(''S'')-''l''-bromo-''l''-methyl-2,2-diphenylcyclopropane with ''n''-butyllithium followed by quenching with
In the same manner the reaction of (+)-(''S'')-''l''-bromo-''l''-methyl-2,2-diphenylcyclopropane with ''n''-butyllithium followed by quenching with  Of recent date are chiral methyllithium compounds:
:
Of recent date are chiral methyllithium compounds:
: The
The
Link
* Large database of Bordwell p''K''a values at daecr1.harvard.ed
Link
{{Authority control Anions Reactive intermediates
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, a carbanion is an anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
in which carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
is trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
(forms three bonds) and bears a formal negative charge (in at least one significant resonance form
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ' ...
).
Formally, a carbanion is the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of a carbon acid:
:deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of alkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in ...
(at an sp3 carbon), alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
(at an sp2 carbon), arenes
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupi ...
(at an sp2 carbon), and alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
(at an sp carbon) are known as alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
, alkenyl (vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
), aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
, and alkynyl (acetylide
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas and , where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure (where R is an organic side c ...
) anions, respectively.
Carbanions have a concentration of electron density at the negatively charged carbon, which, in most cases, reacts efficiently with a variety of electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
s of varying strengths, including carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
s, imines
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
/ iminium salts, halogenating reagents (e.g., ''N''-bromosuccinimide and diiodine), and proton donors. A carbanion is one of several reactive intermediate
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, s ...
s in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
. In organic synthesis, organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s and Grignard reagents
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
are commonly treated and referred to as "carbanions." This is a convenient approximation, although these species are generally clusters or complexes containing highly polar, but still covalent bonds metal–carbon bonds (Mδ+–Cδ−) rather than true carbanions.
Geometry
Absent π delocalization, the negative charge of a carbanion is localized in an sp''x'' hybridized orbital on carbon as alone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. L ...
. As a consequence, ''localized'' alkyl, alkenyl/aryl, and alkynyl carbanions assume trigonal pyramidal, bent, and linear geometries, respectively. By Bent's rule
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization of central atoms in molecules and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows:
The chemical struct ...
, placement of the carbanionic lone pair electrons in an orbital with significant s character is favorable, accounting for the pyramidalized and bent geometries of alkyl and alkenyl carbanions, respectively. Valence shell electron pair repulsion (VSEPR) theory makes similar predictions. This contrasts with carbocations, which have a preference for unoccupied nonbonding orbitals of pure atomic p character, leading to planar and linear geometries, respectively, for alkyl and alkenyl carbocations. However, ''delocalized'' carbanions may deviate from these geometries. Instead of residing in a hybrid orbital, the carbanionic lone pair may instead occupy a p orbital (or an orbital of high p character). A p orbital has a more suitable shape and orientation to overlap with the neighboring π system, resulting in more effective charge delocalization. As a consequence, alkyl carbanions with neighboring conjugating groups (e.g., allylic anions, enolates, nitronates, etc.) are generally planar rather than pyramidized. Likewise, delocalized alkenyl carbanions sometimes favor a linear instead of bent geometry. More often, a bent geometry is still preferred for substituted alkenyl anions, though the linear geometry is only ''slightly'' less stable, resulting in facile equilibration between the (''E'') and (''Z'') isomers of the (bent) anion through a linear transition state. For instance, calculations indicate that the parent vinyl anion, H2C=CH−, has an inversion barrier of , while allenyl anion, H2C=C=H (↔ H2–C≡CH), whose negative charge is stabilized by delocalization, has an inversion barrier of only , reflecting stabilization of the linear transition state by better π delocalization.
However, ''delocalized'' carbanions may deviate from these geometries. Instead of residing in a hybrid orbital, the carbanionic lone pair may instead occupy a p orbital (or an orbital of high p character). A p orbital has a more suitable shape and orientation to overlap with the neighboring π system, resulting in more effective charge delocalization. As a consequence, alkyl carbanions with neighboring conjugating groups (e.g., allylic anions, enolates, nitronates, etc.) are generally planar rather than pyramidized. Likewise, delocalized alkenyl carbanions sometimes favor a linear instead of bent geometry. More often, a bent geometry is still preferred for substituted alkenyl anions, though the linear geometry is only ''slightly'' less stable, resulting in facile equilibration between the (''E'') and (''Z'') isomers of the (bent) anion through a linear transition state. For instance, calculations indicate that the parent vinyl anion, H2C=CH−, has an inversion barrier of , while allenyl anion, H2C=C=H (↔ H2–C≡CH), whose negative charge is stabilized by delocalization, has an inversion barrier of only , reflecting stabilization of the linear transition state by better π delocalization.
Trends and occurrence
Carbanions are typicallynucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
and basic. The basicity and nucleophilicity of carbanions are determined by the substituents on carbon. These include
*the inductive effect
In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.
It is present in a σ (sigma ...
. Electronegative atoms adjacent to the charge will stabilize the charge;
*the extent of conjugation
Conjugation or conjugate may refer to:
Linguistics
*Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
*Complex conjugation, the change ...
of the anion. Resonance effects can stabilize the anion. This is especially true when the anion is stabilized as a result of aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
.
Geometry also affects the orbital hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to ...
of the charge-bearing carbanion. The greater the s-character of the charge-bearing atom, the more stable the anion.
Carbanions, especially ones derived from weak carbon acids that do not benefit sufficiently from the two stabilizing factors listed above, are generally oxygen- and water-sensitive to varying degrees. While some merely degrade and decompose over several weeks or months upon exposure to air, others may react vigorously and exothermically with air almost immediately to spontaneously ignite (pyrophoricity
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
). Among commonly encountered carbanionic reagents in the laboratory, ionic salts of hydrogen cyanide (cyanides
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
) are unusual in being indefinitely stable under dry air and hydrolyzing only very slowly in the presence of moisture.
Organometallic reagents like butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
(hexameric cluster, uLi
Uli may refer to:
*Uli, Iran, a village
*Uli, Anambra, a town in Nigeria
* Uli I of Mali
* Uli (design), by the Igbo people of Nigeria
* Uli figure, from New Ireland, Papua New Guinea
* Uli (food), a rice-based food
* ISO 639 code for the Ulithian ...
sub>6) or methylmagnesium bromide (ether complex, MeMgBr(OEt)2) are often referred to as "carbanions," at least in a retrosynthetic sense. However, they are really clusters or complexes containing a polar covalent bond, though with electron density heavily polarized toward the carbon atom. The more electropositive the attached metal atom, the closer the behavior of the reagent is to that of a true carbanion.
In fact, true carbanions (i.e., a species not attached to a stabilizing covalently bound metal) without electron-withdrawing and/or conjugating substituents are not available in the condensed phase, and these species must be studied in the gas phase. For some time, it was not known whether simple alkyl anions could exist as free species; many theoretical studies predicted that even the methanide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
anion should be an unbound species (i.e., the electron affinity of CH was predicted to be negative). Such a species would decompose immediately by spontaneous ejection of an electron and would therefore be too fleeting to observe directly by mass spectrometry. However, in 1978, the methanide anion was unambiguously synthesized by subjecting ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound ethen ...
to an electric discharge, and the electron affinity (EA) of CH was determined by photoelectron spectroscopy to be +1.8 kcal/mol, making it a bound species, but just barely so. The structure of was found to be pyramidal (C3v) with an H−C−H angle of 108° and inversion
Inversion or inversions may refer to:
Arts
* , a French gay magazine (1924/1925)
* ''Inversion'' (artwork), a 2005 temporary sculpture in Houston, Texas
* Inversion (music), a term with various meanings in music theory and musical set theory
* ...
barrier of 1.3 kcal/mol, while CH was determined to be planar (D3h point group).
Simple primary, secondary and tertiary sp3 carbanions (e.g., ethanide , isopropanide , and ''t''-butanide (CH3)3C−) were subsequently determined to be unbound species (the EAs of CH3CH, (CH3)2CH•, (CH3)3C• are −6, −7.4, −3.6 kcal/mol, respectively) indicating that α substitution is destabilizing. However, relatively modest stabilizing effects can render them bound. For example, cyclopropyl
A cyclopropyl group is a chemical structure derived from cyclopropane, and can participate in organic reactions that constitute cycloadditions and rearrangement organic reactions of cyclopropane. The group has an empirical formula of C3H5 and che ...
and cubyl anions are bound due to increased s character of the lone pair orbital, while neopentyl and phenethyl
Phenethyl alcohol, or 2-phenylethanol, is the organic compound that consists of a phenethyl group (C6H5CH2CH2) attached to OH. It is a colourless liquid that is slightly soluble in water (2 ml/100 ml H2O), but miscible with most organic solvents. ...
anions are also bound, as a result of negative hyperconjugation of the lone pair with the β-substituent (nC → σ*C–C). The same holds true for anions with benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
ic and allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
ic stabilization. Gas-phase carbanions that are sp2 and sp hybridized are much more strongly stabilized and are often prepared directly by gas-phase deprotonation.
In the condensed phase only carbanions that are sufficiently stabilized by delocalization have been isolated as truly ionic species. In 1984, Olmstead and Power
Power most often refers to:
* Power (physics), meaning "rate of doing work"
** Engine power, the power put out by an engine
** Electric power
* Power (social and political), the ability to influence people or events
** Abusive power
Power may a ...
presented the lithium crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
of the triphenylmethanide carbanion from triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmeth ...
, ''n''-butyllithium and 12-crown-4 (which forms a stable complex with lithium cations) at low temperatures:
: Adding ''n''-butyllithium to
Adding ''n''-butyllithium to triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmeth ...
(p''K''a in DMSO of CHPh3 = 30.6) in THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
at low temperatures followed by 12-crown-4 results in a red solution and the salt complex i(12-crown-4)sup>+ Ph3sup>− precipitates at −20 °C. The central C–C bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s are 145 pm with the phenyl ring propellered at an average angle of 31.2°. This propeller shape is less pronounced with a tetramethylammonium counterion. A crystal structure for the analogous diphenylmethanide anion ( i(12-crown-4)sup>+ HPh2sup>−), prepared form diphenylmethane (p''K''a in DMSO of CH2Ph2 = 32.3), was also obtained. However, the attempted isolation of a complex of the benzyl anion H2Phsup>− from toluene (p''K''a in DMSO of CH3Ph ≈ 43) was unsuccessful, due to rapid reaction of the formed anion with the THF solvent. The free benzyl anion has also been generated in the solution phase by pulse radiolysis of dibenzylmercury.
Early in 1904 and 1917, Schlenk prepared two red-colored salts, formulated as Me4sup>+ Ph3sup>− and Me4sup>+ H2Phsup>−, respectively, by metathesis of the corresponding organosodium reagent with tetramethylammonium chloride. Since tetramethylammonium cations cannot form a chemical bond to the carbanionic center, these species are believed to contain free carbanions. While the structure of the former was verified by X-ray crystallography almost a century later, the instability of the latter has so far precluded structural verification. The reaction of the putative " Me4sup>+ H2Phsup>−" with water was reported to liberate toluene and tetramethylammonium hydroxide and provides indirect evidence for the claimed formulation.
One tool for the detection of carbanions in solution is proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the struct ...
. A spectrum of cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca ...
in DMSO shows four vinylic protons at 6.5 ppm and two methylene bridge protons at 3 ppm whereas the cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
anion has a single resonance at 5.50 ppm. The use of 6Li and 7Li NMR has provided structural and reactivity data for a variety of organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
species.
Carbon acids
Any compound containing hydrogen can, in principle, undergo deprotonation to form its conjugate base. A compound is a carbon acid if deprotonation results in loss of a proton from a carbon atom. Compared to compounds typically considered to be acids (e.g.,mineral acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Cha ...
s like nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, or carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
s like acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
), carbon acids are typically many orders of magnitude weaker, although exceptions exist (see below). For example, benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
is not an acid in the classical Arrhenius sense, since its aqueous solutions are neutral. Nevertheless, it is very weak Brønsted acid with an estimated p''K''a of 49 which may undergo deprotonation in the presence of a superbase like the Lochmann–Schlosser base ( ''n''-butyllithium and potassium ''t''-butoxide). As conjugate acid–base pairs, the factors that determine the relative stability of carbanions also determine the ordering of the p''K''a values of the corresponding carbon acids. Furthermore, p''K''a values allow the prediction of whether a proton transfer process will be thermodynamically favorable: In order for the deprotonation of an acidic species HA with base B− to be thermodynamically favorable (''K'' > 1), the relationship p''K''a(BH) > p''K''a(AH) must hold.
These values below are p''K''a values determined in dimethylsulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and ...
(DMSO), which has a broader useful range (~0 to ~35) than values determined in water (~0 to ~14) and better reflect the basicity of the carbanions in typical organic solvents. Values below less than 0 or greater than 35 are indirectly estimated; hence, the numerical accuracy of these values is limited. Aqueous p''K''a values are also commonly encountered in the literature, particularly in the context of biochemistry and enzymology. Moreover, aqueous values are often given in introductory organic chemistry textbooks for pedagogical reasons, although the issue of solvent dependence is often glossed over. In general, p''K''a values in water and organic solvent diverge significantly when the anion is capable of hydrogen bonding. For instance, in the case of water, the values differ dramatically: the p''K''a in water of water is 14.0, while the p''K''a in DMSO of water is 31.4, reflecting the differing ability of water and DMSO to stabilize the hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
anion. On the other hand, for cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca ...
, the numerical values are comparable: the p''K''a in water is 15, while the p''K''a in DMSO is 18.
:
::Note that acetic acid, ammonia, aniline, ethanol, and hydrogen chloride are not carbon acids, but are common acids shown for comparison.
As indicated by the examples above, acidity increases (p''K''a decreases) when the negative charge is delocalized. This effect occurs when the substituents on the carbanion are unsaturated and/or electronegative. Although carbon acids are generally thought of as acids that are much weaker than "classical" Brønsted acids like acetic acid or phenol, the cumulative (additive) effect of several electron accepting substituents can lead to acids that are as strong or stronger than the inorganic mineral acids. For example, trinitromethane
Trinitromethane, also referred to as nitroform, is a nitroalkane and oxidizer with chemical formula HC(NO2)3. It was first obtained in 1857 as the ammonium salt by the Russian chemist Leon Nikolaevich Shishkov (1830–1908). In 1900, it was dis ...
HC(NO2)3, tricyanomethane HC(CN)3, pentacyanocyclopentadiene C5(CN)5H, and fulminic acid
Fulminic acid is an acid with the formula HCNO, more specifically . It is an isomer of isocyanic acid () and of its elusive tautomer, cyanic acid (), and also of isofulminic acid ().
Fulminate is the anion or any of its salts. For historic ...
HCNO, are all strong acids with aqueous p''K''a values that indicate complete or nearly complete proton transfer to water. Triflidic acid
Triflidic acid (''IUPAC name'': tris trifluoromethyl)sulfonylethane, ''abbreviated formula'': Tf3CH) is an organic superacid. It is one of the strongest known carbon acids and is among the strongest Brønsted acids in general, with an acidity ex ...
, with three strongly electron-withdrawing triflyl
In organic chemistry, the triflyl group (systematic name: trifluoromethanesulfonyl group) is a functional group with the formula and structure
A structure is an arrangement and organization of interrelated elements in a material object or sy ...
groups, has an estimated p''K''a well below −10. On the other end of the scale, hydrocarbons bearing only alkyl groups are thought to have p''K''a values in the range of 55 to 65. The range of acid dissociation constants for carbon acids thus spans over 70 orders of magnitude.
The acidity of the α-hydrogen in carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
compounds enables these compounds to participate in synthetically important C–C bond-forming reactions including the aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two ...
and Michael addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
.
Chiral carbanions
With themolecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that dete ...
for a carbanion described as a trigonal pyramid
In geometry, a pyramid () is a polyhedron formed by connecting a polygonal base and a point, called the apex. Each base edge and apex form a triangle, called a ''lateral face''. It is a conic solid with polygonal base. A pyramid with an base ...
the question is whether or not carbanions can display chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
, because if the activation barrier for inversion of this geometry is too low any attempt at introducing chirality will end in racemization In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. conta ...
, similar to the nitrogen inversion In chemistry, pyramidal inversion (also umbrella inversion) is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion pass ...
. However, solid evidence exists that carbanions can indeed be chiral for example in research carried out with certain organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
compounds.
The first ever evidence for the existence of chiral organolithium compounds was obtained in 1950. Reaction of chiral 2-iodooctane with ''s''-butyllithium in petroleum ether
Petroleum ether is the petroleum fraction consisting of aliphatic hydrocarbons and boiling in the range 35–60 °C, and commonly used as a laboratory solvent. Despite the name, petroleum ether is not classified as an ether; the term is used ...
at −70 °C followed by reaction with dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimates directly from the solid state to the gas state. It is used primarily ...
yielded mostly racemic 2-methylbutyric acid but also an amount of optically active
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
2-methyloctanoic acid, which could only have formed from likewise optically active 2-methylheptyllithium with the carbon atom linked to lithium the carbanion:
: On heating the reaction to 0 °C the optical activity is lost. More evidence followed in the 1960s. A reaction of the ''cis'' isomer of 2-methylcyclopropyl bromide with ''s''-butyllithium again followed by
On heating the reaction to 0 °C the optical activity is lost. More evidence followed in the 1960s. A reaction of the ''cis'' isomer of 2-methylcyclopropyl bromide with ''s''-butyllithium again followed by carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylatio ...
with dry ice yielded ''cis''-2-methylcyclopropylcarboxylic acid. The formation of the ''trans'' isomer would have indicated that the intermediate carbanion was unstable.
: In the same manner the reaction of (+)-(''S'')-''l''-bromo-''l''-methyl-2,2-diphenylcyclopropane with ''n''-butyllithium followed by quenching with
In the same manner the reaction of (+)-(''S'')-''l''-bromo-''l''-methyl-2,2-diphenylcyclopropane with ''n''-butyllithium followed by quenching with methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
resulted in product with retention of configuration
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule f ...
:
: Of recent date are chiral methyllithium compounds:
:
Of recent date are chiral methyllithium compounds:
: The
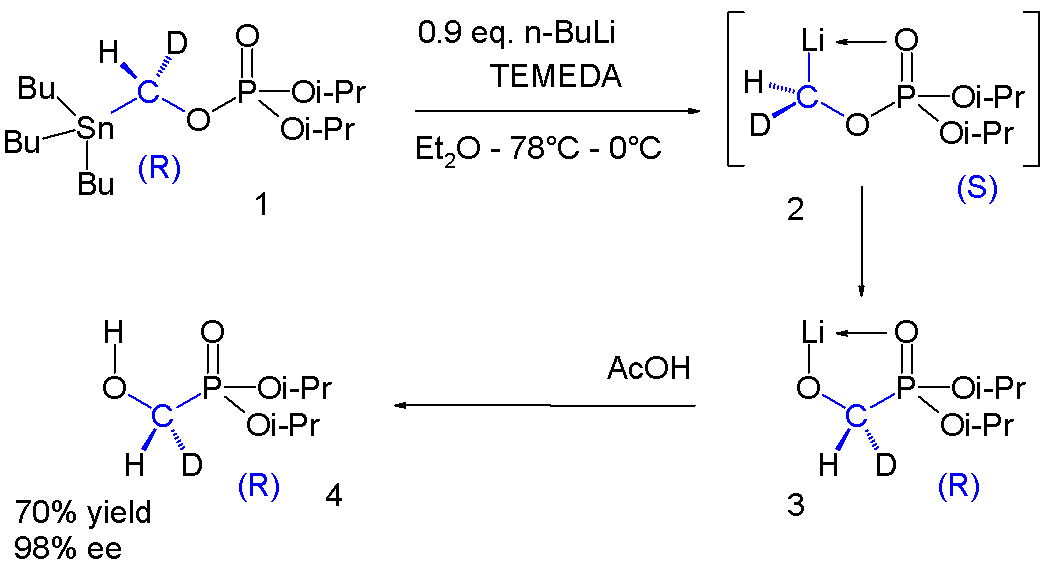
The phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
1 contains a chiral group with a hydrogen and a deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
substituent. The stannyl
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered ...
group is replaced by lithium to intermediate 2 which undergoes a phosphate–phosphorane rearrangement to phosphorane
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the hypothetical molecule PH5. The derivative pentaphenylph ...
3 which on reaction with acetic acid gives alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
4. Once again in the range of −78 °C to 0 °C the chirality is preserved in this reaction sequence. (Enantioselectivity
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
was determined by NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fi ...
after derivatization with Mosher's acid
Mosher's acid, or α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) is a carboxylic acid which was first used by Harry Stone Mosher as a chiral derivatizing agent. It is a chiral molecule, consisting of ''R'' and ''S'' enantiomers.
Applicat ...
.)
History
A carbanionic structure first made an appearance in the reaction mechanism for the benzoin condensation as correctly proposed by Clarke and Arthur Lapworth in 1907. In 1904Wilhelm Schlenk
Wilhelm may refer to:
People and fictional characters
* William Charles John Pitcher, costume designer known professionally as "Wilhelm"
* Wilhelm (name), a list of people and fictional characters with the given name or surname
Other uses
* Mount ...
prepared Ph3C− in a quest for pentavalent nitrogen (from tetramethylammonium chloride
Tetramethylammonium chloride is one of the simplest quaternary ammonium salts, with four methyl groups tetrahedrally attached to the central N. The chemical formula (CH3)4N+Cl− is often abbreviated further as Me4N+Cl−. It is a hygroscopic colo ...
and
Ph3CNa) and in 1914 he demonstrated how triarylmethyl radicals could be reduced to carbanions by alkali metals The phrase carbanion was introduced by Wallis and Adams in 1933 as the negatively charged counterpart of the carbonium ion
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms.
The ...
See also
*Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
*Enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
s
*Nitrile anion Nitrile anions is jargon from the organic product resulting from the deprotonation of alkylnitriles. The proton(s) α to the nitrile group are sufficiently acidic that they undergo deprotonation by strong bases, usually lithium-derived. The produc ...
References
External links
* Large database of Bordwell p''K''a values at www.chem.wisc.edLink
* Large database of Bordwell p''K''a values at daecr1.harvard.ed
Link
{{Authority control Anions Reactive intermediates