Bohr effect on:
[Wikipedia]
[Google]
[Amazon]
 The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist
 In the early 1900s, Christian Bohr was a professor at the
In the early 1900s, Christian Bohr was a professor at the
CO2 + H2O <=> H2CO3 <=> H+ + HCO3^-
Although this reaction usually proceeds very slowly, the enzyme carbonic anhydrase (which is present in red blood cells) drastically speeds up the conversion to bicarbonate and protons. This causes the pH of the blood to decrease, which promotes the dissociation of oxygen from haemoglobin, and allows the surrounding tissues to obtain enough oxygen to meet their demands. In areas where oxygen concentration is high, such as the lungs, binding of oxygen causes haemoglobin to release protons, which recombine with bicarbonate to eliminate carbon dioxide during 
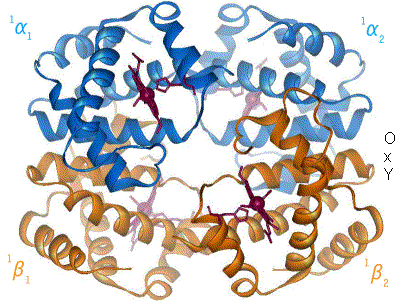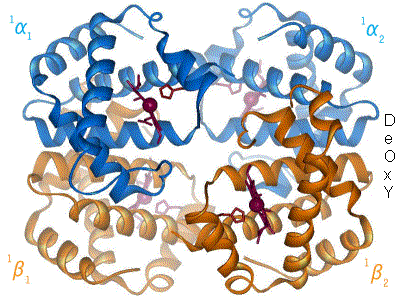 The Bohr effect hinges around allosteric interactions between the
The Bohr effect hinges around allosteric interactions between the
R-NH2 + CO2 <=> R-NH-COO^- + H+
CO2 forms carbamates more frequently with the T state, which helps to stabilize this conformation. The process also creates protons, meaning that the formation of carbamates also contributes to the strengthening of ionic interactions, further stabilizing the T state.

Impact of training
{{Respiratory physiology Respiratory physiology
 The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr
Christian Harald Lauritz Peter Emil Bohr (1855–1911) was a Danish physician, father of the physicist and Nobel laureate Niels Bohr, as well as the mathematician and football player Harald Bohr and grandfather of another physicist and Nobel lau ...
. Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
's oxygen binding affinity (see oxygen–haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
or the pH of the environment. Since carbon dioxide reacts with water to form carbonic acid, an increase in CO2 results in a decrease in blood pH, resulting in hemoglobin proteins releasing their load of oxygen. Conversely, a decrease in carbon dioxide provokes an increase in pH, which results in hemoglobin picking up more oxygen.
Experimental discovery
 In the early 1900s, Christian Bohr was a professor at the
In the early 1900s, Christian Bohr was a professor at the University of Copenhagen
The University of Copenhagen ( da, Københavns Universitet, KU) is a prestigious public research university in Copenhagen, Denmark. Founded in 1479, the University of Copenhagen is the second-oldest university in Scandinavia after Uppsala Unive ...
in Denmark, already well known for his work in the field of respiratory physiology. He had spent the last two decades studying the solubility of oxygen, carbon dioxide, and other gases in various liquids, and had conducted extensive research on haemoglobin and its affinity for oxygen. In 1903, he began working closely with Karl Hasselbalch and August Krogh
Schack August Steenberg Krogh (15 November 1874 – 13 September 1949) was a Danish professor at the department of zoophysiology at the University of Copenhagen from 1916 to 1945. He contributed a number of fundamental discoveries within severa ...
, two of his associates at the university, in an attempt to experimentally replicate the work of Gustav von Hüfner, using whole blood instead of haemoglobin solution. Hüfner had suggested that the oxygen-haemoglobin binding curve was hyperbolic
Hyperbolic is an adjective describing something that resembles or pertains to a hyperbola (a curve), to hyperbole (an overstatement or exaggeration), or to hyperbolic geometry.
The following phenomena are described as ''hyperbolic'' because they ...
in shape, but after extensive experimentation, the Copenhagen group determined that the curve was in fact sigmoidal. Furthermore, in the process of plotting out numerous dissociation curves, it soon became apparent that high partial pressures of carbon dioxide caused the curves to shift to the right. Further experimentation while varying the CO2 concentration quickly provided conclusive evidence, confirming the existence of what would soon become known as the Bohr effect.
Controversy
There is some more debate over whether Bohr was actually the first to discover the relationship between CO2 and oxygen affinity, or whether the Russian physiologist beat him to it, allegedly discovering the effect in 1898, six years before Bohr. While this has never been proven, Verigo did in fact publish a paper on the haemoglobin-CO2 relationship in 1892. His proposed model was flawed, and Bohr harshly criticized it in his own publications. Another challenge to Bohr's discovery comes from within his lab. Though Bohr was quick to take full credit, his associate Krogh, who invented the apparatus used to measure gas concentrations in the experiments, maintained throughout his life that he himself had actually been the first to demonstrate the effect. Though there is some evidence to support this, retroactively changing the name of a well-known phenomenon would be extremely impractical, so it remains known as the Bohr effect.Physiological role
The Bohr effect increases the efficiency of oxygen transportation through the blood. After hemoglobin binds to oxygen in the lungs due to the high oxygen concentrations, the Bohr effect facilitates its release in the tissues, particularly those tissues in most need of oxygen. When a tissue's metabolic rate increases, so does its carbon dioxide waste production. When released into the bloodstream, carbon dioxide formsbicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochem ...
and protons through the following reaction:
:exhalation
Exhalation (or expiration) is the flow of the breath out of an organism. In animals, it is the movement of air from the lungs out of the airways, to the external environment during breathing.
This happens due to elastic properties of the lungs, ...
. These opposing protonation and deprotonation reactions occur in equilibrium resulting in little overall change in blood pH.
The Bohr effect enables the body to adapt to changing conditions and makes it possible to supply extra oxygen to tissues that need it the most. For example, when muscles
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of musc ...
are undergoing strenuous activity, they require large amounts of oxygen to conduct cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
, which generates CO2 (and therefore HCO3− and H+) as byproducts. These waste products lower the pH of the blood, which increases oxygen delivery to the active muscles. Carbon dioxide is not the only molecule that can trigger the Bohr effect. If muscle cells aren't receiving enough oxygen for cellular respiration, they resort to lactic acid fermentation
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate, which is lactic acid i ...
, which releases lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as nat ...
as a byproduct. This increases the acidity of the blood far more than CO2 alone, which reflects the cells' even greater need for oxygen. In fact, under anaerobic conditions, muscles generate lactic acid so quickly that pH of the blood passing through the muscles
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of musc ...
will drop to around 7.2, which causes haemoglobin to begin releasing roughly 10% more oxygen.
Strength of the effect and body size
The magnitude of the Bohr effect is usually given by the slope of the vs curve where, P50 refers to the partial pressure of oxygen when 50% of haemoglobin's binding sites are occupied. The slope is denoted: where denotes change. That is, denotes the change in and the change in . Bohr effect strength exhibits an inverse relationship with the size of an organism: the magnitude increases as size and weight decreases. For example, mice possess a very strong Bohr effect, with a value of -0.96, which requires relatively minor changes in H+ or CO2 concentrations, whileelephant
Elephants are the largest existing land animals. Three living species are currently recognised: the African bush elephant, the African forest elephant, and the Asian elephant. They are the only surviving members of the family Elephantidae ...
s require much larger changes in concentration to achieve a much weaker effect .
Mechanism
Allosteric interactions
 The Bohr effect hinges around allosteric interactions between the
The Bohr effect hinges around allosteric interactions between the heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
s of the haemoglobin tetramer
A tetramer () ('' tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
, a mechanism first proposed by Max Perutz in 1970. Haemoglobin exists in two conformations: a high-affinity R state and a low-affinity T state. When oxygen concentration levels are high, as in the lungs, the R state is favored, enabling the maximum amount of oxygen to be bound to the hemes. In the capillaries, where oxygen concentration levels are lower, the T state is favored, in order to facilitate the delivery of oxygen to the tissues. The Bohr effect is dependent on this allostery, as increases in CO2 and H+ help stabilize the T state and ensure greater oxygen delivery to muscles during periods of elevated cellular respiration. This is evidenced by the fact that myoglobin, a monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
with no allostery, does not exhibit the Bohr effect. Haemoglobin mutants with weaker allostery may exhibit a reduced Bohr effect. For example, in Hiroshima variant haemoglobinopathy, allostery in haemoglobin is reduced, and the Bohr effect is diminished. As a result, during periods of exercise, the mutant haemoglobin has a higher affinity for oxygen and tissue may suffer minor oxygen starvation.
T-state stabilization
When hemoglobin is in its T state, the N-terminal amino groups of the α-subunits and theC-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
of the β-subunits are protonated, giving them a positive charge and allowing these residues to participate in ionic interactions with carboxyl groups on nearby residues. These interactions help hold the haemoglobin in the T state. Decreases in pH (increases in acidity) stabilize this state even more, since a decrease in pH makes these residues even more likely to be protonated, strengthening the ionic interactions. In the R state, the ionic pairings are absent, meaning that the R state's stability increases when the pH increases, as these residues are less likely to stay protonated in a more basic environment. The Bohr effect works by simultaneously destabilizing the high-affinity R state and stabilizing the low-affinity T state, which leads to an overall decrease in oxygen affinity. This can be visualized on an oxygen-haemoglobin dissociation curve by shifting the whole curve to the right.
Carbon dioxide can also react directly with the N-terminal amino groups to form carbamates
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
, according to the following reaction:
: Special cases

Marine mammals
An exception to the otherwise well-supported link between animal body size and the sensitivity of its haemoglobin to changes in pH was discovered in 1961. Based on their size and weight, manymarine mammal
Marine mammals are aquatic mammals that rely on the ocean and other marine ecosystems for their existence. They include animals such as seals, whales, manatees, sea otters and polar bears. They are an informal group, unified only by their ...
s were hypothesized to have a very low, almost negligible Bohr effect. However, when their blood was examined, this was not the case. Humpback whale
The humpback whale (''Megaptera novaeangliae'') is a species of baleen whale. It is a rorqual (a member of the family Balaenopteridae) and is the only species in the genus ''Megaptera''. Adults range in length from and weigh up to . The hu ...
s weighing 41,000 kilograms had an observed value of 0.82, which is roughly equivalent to the Bohr effect magnitude in a 0.57 kg guinea pig
The guinea pig or domestic guinea pig (''Cavia porcellus''), also known as the cavy or domestic cavy (), is a species of rodent belonging to the genus '' Cavia'' in the family Caviidae. Breeders tend to use the word ''cavy'' to describe the ...
. This extremely strong Bohr effect is hypothesized to be one of marine mammals' many adaptations for deep, long dives, as it allows for virtually all of the bound oxygen on haemoglobin to dissociate and supply the whale's body while it is underwater. Examination of other marine mammal species supports this. In pilot whale
Pilot whales are cetaceans belonging to the genus ''Globicephala''. The two extant species are the long-finned pilot whale (''G. melas'') and the short-finned pilot whale (''G. macrorhynchus''). The two are not readily distinguishable at sea, ...
s and porpoises, which are primarily surface feeders and seldom dive for more than a few minutes, the was 0.52, comparable to a cow
Cattle (''Bos taurus'') are large, domesticated, cloven-hooved, herbivores. They are a prominent modern member of the subfamily Bovinae and the most widespread species of the genus ''Bos''. Adult females are referred to as cows and adult ma ...
, which is much closer to the expected Bohr effect magnitude for animals of their size.
Carbon monoxide
Another special case of the Bohr effect occurs whencarbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
is present. This molecule serves as a competitive inhibitor
Competitive inhibition is interruption of a chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for binding or bonding. Any metabolic or chemical messenger system can potentially be affected b ...
for oxygen, and binds to haemoglobin to form carboxyhaemoglobin
Carboxyhemoglobin (carboxyhaemoglobin BrE) (symbol COHb or HbCO) is a stable complex of carbon monoxide and hemoglobin (Hb) that forms in red blood cells upon contact with carbon monoxide. Carboxyhemoglobin is often mistaken for the compound form ...
. Haemoglobin's affinity for CO is about 210 times stronger than its affinity for O2, meaning that it is very unlikely to dissociate, and once bound, it blocks the binding of O2 to that subunit. At the same time, CO is structurally similar enough to O2 to cause carboxyhemoglobin to favor the R state, raising the oxygen affinity of the remaining unoccupied subunits. This combination significantly reduces the delivery of oxygen to the tissues of the body, which is what makes carbon monoxide so toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
. This toxicity is reduced slightly by an increase in the strength of the Bohr effect in the presence of carboxyhemoglobin. This increase is ultimately due to differences in interactions between heme groups in carboxyhemoglobin relative to oxygenated hemoglobin. It is most pronounced when the oxygen concentration is extremely low, as a last-ditch effort when the need for oxygen delivery becomes critical. However, the physiological implications of this phenomenon remain unclear.
See also
* Allosteric regulation * Haldane effect *Root effect
The Root effect is a physiological phenomenon that occurs in fish hemoglobin, named after its discoverer R. W. Root. It is the phenomenon where an increased proton or carbon dioxide concentration (lower pH) lowers hemoglobin's affinity and carry ...
* Chloride shift
References
External links
Impact of training
{{Respiratory physiology Respiratory physiology