Biological aspects of fluorine on:
[Wikipedia]
[Google]
[Amazon]
 Fluorine may interact with biological systems in the form of fluorine-containing compounds. Though elemental fluorine (F2) is very rare in everyday life, fluorine-containing compounds such as
Fluorine may interact with biological systems in the form of fluorine-containing compounds. Though elemental fluorine (F2) is very rare in everyday life, fluorine-containing compounds such as
 Of all commercialized pharmaceutical drugs, twenty percent contain fluorine, including important drugs in many different pharmaceutical classes. Fluorine is often added to drug molecules during
Of all commercialized pharmaceutical drugs, twenty percent contain fluorine, including important drugs in many different pharmaceutical classes. Fluorine is often added to drug molecules during
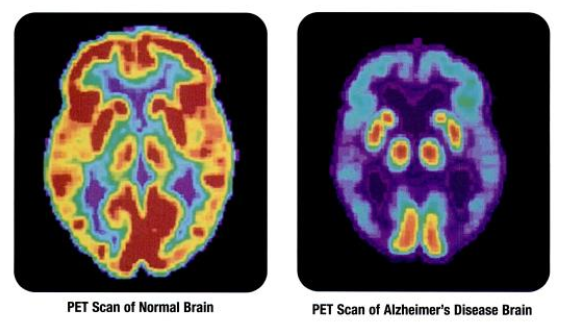 Compounds containing fluorine-18, a radioactive isotope that emits positrons, are often used in positron emission tomography (PET) scanning, because the isotope's half-life of about 110 minutes is usefully long by positron-emitter standards. One such
Compounds containing fluorine-18, a radioactive isotope that emits positrons, are often used in positron emission tomography (PET) scanning, because the isotope's half-life of about 110 minutes is usefully long by positron-emitter standards. One such
 Blood substitutes are the subject of research because the demand for blood transfusions grows faster than donations. In some scenarios, artificial blood may be more convenient or safe. Because fluorocarbons do not normally mix with water, they must be mixed into emulsions (small droplets of perfluorocarbon suspended in water) in order to be used as blood. One such product, Oxycyte, has been through initial clinical trials.
Possible medical uses of liquid breathing (which uses pure perfluorocarbon liquid, not a water emulsion) involve assistance for premature babies or for burn patients (if normal lung function is compromised). Both partial and complete filling of the lungs have been considered, although only the former has undergone any significant tests in humans. Several animal tests have been performed and there have been some human partial liquid ventilation trials. One effort, by Alliance Pharmaceuticals, reached clinical trials but was abandoned because of insufficient advantage compared to other therapies.
Nanocrystals represent a possible method of delivering water- or fat-soluble drugs within a perfluorochemical fluid. The use of these particles is being developed to help treat babies with damaged lungs.
Perfluorocarbons are banned from sports, where they may be used to increase oxygen use for endurance athletes. One cyclist, Mauro Gianetti, was investigated after a near-fatality where PFC use was suspected. Other posited applications include deep-sea diving and space travel, applications that both require total, not partial, liquid ventilation. The 1989 film ''
Blood substitutes are the subject of research because the demand for blood transfusions grows faster than donations. In some scenarios, artificial blood may be more convenient or safe. Because fluorocarbons do not normally mix with water, they must be mixed into emulsions (small droplets of perfluorocarbon suspended in water) in order to be used as blood. One such product, Oxycyte, has been through initial clinical trials.
Possible medical uses of liquid breathing (which uses pure perfluorocarbon liquid, not a water emulsion) involve assistance for premature babies or for burn patients (if normal lung function is compromised). Both partial and complete filling of the lungs have been considered, although only the former has undergone any significant tests in humans. Several animal tests have been performed and there have been some human partial liquid ventilation trials. One effort, by Alliance Pharmaceuticals, reached clinical trials but was abandoned because of insufficient advantage compared to other therapies.
Nanocrystals represent a possible method of delivering water- or fat-soluble drugs within a perfluorochemical fluid. The use of these particles is being developed to help treat babies with damaged lungs.
Perfluorocarbons are banned from sports, where they may be used to increase oxygen use for endurance athletes. One cyclist, Mauro Gianetti, was investigated after a near-fatality where PFC use was suspected. Other posited applications include deep-sea diving and space travel, applications that both require total, not partial, liquid ventilation. The 1989 film ''
 Biologically synthesized organofluorines are few in number, although some are widely produced. The most common example is
Biologically synthesized organofluorines are few in number, although some are widely produced. The most common example is
 Fluorine may interact with biological systems in the form of fluorine-containing compounds. Though elemental fluorine (F2) is very rare in everyday life, fluorine-containing compounds such as
Fluorine may interact with biological systems in the form of fluorine-containing compounds. Though elemental fluorine (F2) is very rare in everyday life, fluorine-containing compounds such as fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs sca ...
occur naturally as minerals. Naturally occurring organofluorine
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, ref ...
compounds are extremely rare. Man-made fluoride compounds are common and are used in medicines, pesticides, and materials. Twenty percent of all commercialized pharmaceuticals contain fluorine, including Lipitor
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
Common ...
and Prozac
Fluoxetine, sold under the brand names Prozac and Sarafem, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used for the treatment of major depressive disorder, obsessive–compulsive disorde ...
.G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick "Fluorine Compounds, Organic" in "Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. In many contexts, fluorine-containing compounds are harmless or even beneficial to living organisms; in others, they are toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
.
Aside from their use in medicine, man-made fluorinated compounds have also played a role in several noteworthy environmental concerns. Chlorofluorocarbons
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propa ...
(CFCs), once major components of numerous commercial aerosol products, have proven damaging to Earth's ozone layer and resulted in the wide-reaching Montreal Protocol
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force o ...
; though in truth the chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
in CFCs is the destructive actor, fluorine is an important part of these molecules because it makes them very stable and long-lived. Similarly, the stability of many organofluorine compounds has raised the issue of biopersistence. Long-lived molecules from waterproofing sprays, for example PFOA
Perfluorooctanoic acid (PFOA; conjugate base perfluorooctanoate; also known colloquially as C8, for its 8 carbon chain structure) is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes a ...
and PFOS, are found worldwide in the tissues of wildlife and humans, including newborn children.
Fluorine biology is also relevant to a number of cutting-edge technologies. PFCs (perfluorocarbons
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
) are capable of holding enough oxygen to support human liquid breathing
Liquid breathing is a form of respiration in which a normally air-breathing organism breathes an oxygen-rich liquid (such as a perfluorocarbon), rather than breathing air.
By selecting a liquid that is capable of holding large amounts of oxy ...
. Organofluorine in the form of its radioisotope 18F is also at the heart of a modern medical imaging technique known as positron emission tomography (PET). A PET scan produces three-dimensional colored images of parts of the body that use a lot of sugar, particularly the brain or tumors.
Dental care
Since the mid-20th century, it has been discerned from population studies (though incompletely understood) that fluoride reducestooth decay
Tooth decay, also known as cavities or caries, is the breakdown of teeth due to acids produced by bacteria. The cavities may be a number of different colors from yellow to black. Symptoms may include pain and difficulty with eating. Complicatio ...
. Initially, researchers hypothesized that fluoride helped by converting tooth enamel
Tooth enamel is one of the four major tissues that make up the tooth in humans and many other animals, including some species of fish. It makes up the normally visible part of the tooth, covering the crown. The other major tissues are dentin, ...
from the more acid-soluble mineral hydroxyapatite to the less acid-soluble mineral fluorapatite. However, more recent studies showed no difference in the frequency of caries
Tooth decay, also known as cavities or caries, is the breakdown of teeth due to acids produced by bacteria. The cavities may be a number of different colors from yellow to black. Symptoms may include pain and difficulty with eating. Complicatio ...
(cavities) between teeth that were pre-fluoridated to different degrees. Current thinking is that fluoride prevents cavities primarily by helping teeth that are in the very early stages of tooth decay.
When teeth begin to decay from the acid produced by sugar-consuming bacteria, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
is lost (''demineralization''). However, teeth have a limited ability to recover calcium if decay is not too far advanced (''remineralization''). Fluoride appears to reduce demineralization and increase remineralization. Also, there is some evidence that fluoride interferes with the bacteria that consume sugars in the mouth and make tooth-destroying acids. In any case, it is only the fluoride that is directly present in the mouth ( topical treatment) that prevents cavities; fluoride ions that are swallowed do not benefit the teeth.
Water fluoridation
Water fluoridation is the controlled adjustment of fluoride to a public water supply solely to reduce tooth decay. Fluoridated water contains fluoride at a level that is effective for preventing cavities; this can occur naturally or by adding ...
is the controlled addition of fluoride to a public water supply
In public relations and communication science, publics are groups of individual people, and the public (a.k.a. the general public) is the totality of such groupings. This is a different concept to the sociological concept of the ''Öffentlichke ...
in an effort to reduce tooth decay in people who drink the water. Its use began in the 1940s, following studies of children in a region where water is naturally fluoridated. It is now used widely in public water systems in the United States and some other parts of the world, such that about two-thirds of the U.S. population is exposed to fluoridated water supplies and about 5.7% of people worldwide. Although the best available evidence shows no association with adverse effects other than fluorosis ( dental and, in worse cases, skeletal), most of which is mild, water fluoridation has been contentious for ethical, safety, and efficacy reasons, and opposition to water fluoridation exists despite its widespread support by public health
Public health is "the science and art of preventing disease, prolonging life and promoting health through the organized efforts and informed choices of society, organizations, public and private, communities and individuals". Analyzing the det ...
organizations. The benefits of water fluoridation have lessened recently, presumably because of the availability of fluoride in other forms, but are still measurable, particularly for low-income groups. Systematic reviews in 2000 and 2007 showed significant reduction of cavities in children exposed to water fluoridation. Summary:
Sodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is used in trace amounts in the fluoridation of drinking water, in toothpaste, in metallurgy, and as a flux. It is a colorless or white solid that is readily soluble in water. I ...
, tin difluoride, and, most commonly, sodium monofluorophosphate
Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.Klaus Schrödter ...
, are used in toothpaste
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, ...
. In 1955, the first fluoride toothpaste was introduced in the United States. Now, almost all toothpaste in developed countries is fluoridated. For example, 95% of European toothpaste contains fluoride. Gels and foams are often advised for special patient groups, particularly those undergoing radiation therapy to the head (cancer patients). The patient receives a four-minute application of a high amount of fluoride. Varnishes, which can be more quickly applied, exist and perform a similar function. Fluoride is also often present in prescription and non-prescription mouthwash
Mouthwash, mouth rinse, oral rinse, or mouth bath is a liquid which is held in the mouth passively or swilled around the mouth by contraction of the perioral muscles and/or movement of the head, and may be gargled, where the head is tilted back ...
es and is a trace component of foods manufactured using fluoridated water supplies.
Medical applications
Pharmaceuticals
 Of all commercialized pharmaceutical drugs, twenty percent contain fluorine, including important drugs in many different pharmaceutical classes. Fluorine is often added to drug molecules during
Of all commercialized pharmaceutical drugs, twenty percent contain fluorine, including important drugs in many different pharmaceutical classes. Fluorine is often added to drug molecules during drug design
Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that acti ...
, as even a single atom can greatly change the chemical properties of the molecule in desirable ways.
Because of the considerable stability of the carbon–fluorine bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F ...
, many drugs are fluorinated to delay their metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
, which is the chemical process in which the drugs are turned into compounds that allows them to be eliminated. This prolongs their half-lives
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
and allows for longer times between dosing and activation. For example, an aromatic ring
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
may prevent the metabolism of a drug, but this presents a safety problem, because some aromatic compounds are metabolized in the body into poisonous epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
s by the organism's native enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s. Substituting a fluorine into a ''para'' position, however, protects the aromatic ring and prevents the epoxide from being produced.
Adding fluorine to biologically active organic compounds increases their lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lip ...
(ability to dissolve in fats), because the carbon–fluorine bond is even more hydrophobic than the carbon–hydrogen bond
In chemistry, the carbon-hydrogen bond ( bond) is a chemical bond between carbon and hydrogen atoms that can be found in many organic compounds. This bond is a covalent, single bond, meaning that carbon shares its outer valence electrons with up t ...
. This effect often increases a drug's bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
because of increased cell membrane penetration. Although the potential of fluorine being released in a fluoride leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
depends on its position in the molecule, organofluorides are generally very stable, since the carbon–fluorine bond is strong.
Fluorines also find their uses in common mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances ( electrolyte balance and fluid balance). The primary ...
s, a class of drugs that increase the blood pressure. Adding a fluorine increases both its medical power and anti-inflammatory effects. Fluorine-containing fludrocortisone
Fludrocortisone, sold under the brand name Florinef, among others, is a corticosteroid used to treat adrenogenital syndrome, postural hypotension, and adrenal insufficiency. In adrenal insufficiency, it is generally taken together with hydroc ...
is one of the most common of these drugs. Dexamethasone
Dexamethasone is a glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive lung disease, croup, brain swelling, eye pain following eye surgery, superior vena ...
and triamcinolone
Triamcinolone is a glucocorticoid used to treat certain skin diseases, allergies, and rheumatic disorders among others. It is also used to prevent worsening of asthma and COPD. It can be taken in various ways including by mouth, injection i ...
, which are among the most potent of the related synthetic corticosteroid class of drugs, contain fluorine as well.
Several inhaled general anesthetic
An anesthetic (American English) or anaesthetic (British English; see spelling differences) is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two ...
agents, including the most commonly used inhaled agents, also contain fluorine. The first fluorinated anesthetic agent, halothane
Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful i ...
, proved to be much safer (neither explosive nor flammable) and longer-lasting than those previously used. Modern fluorinated anesthetics are longer-lasting still and almost insoluble in blood, which accelerates the awakening. Examples include sevoflurane
Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflura ...
, desflurane, enflurane
Enflurane (2-chloro-1,1,2-trifluoroethyl difluoromethyl ether) is a halogenated ether. Developed by Ross Terrell in 1963, it was first used clinically in 1966. It was increasingly used for inhalational anesthesia during the 1970s and 1980s but ...
, and isoflurane
Isoflurane, sold under the brand name Forane among others, is a general anesthetic. It can be used to start or maintain anesthesia; however, other medications are often used to start anesthesia rather than isoflurane, due to airway irritation w ...
, all hydrofluorocarbon
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air condi ...
derivatives.
Prior to the 1980s, antidepressants altered not only serotonin uptake but also the uptake of altered norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as both a hormone and neurotransmitter. The name "noradrenaline" (from Latin '' ad' ...
; the latter caused most of the side effects of antidepressants. The first drug to alter only the serotonin uptake was Prozac
Fluoxetine, sold under the brand names Prozac and Sarafem, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used for the treatment of major depressive disorder, obsessive–compulsive disorde ...
; it gave birth to the extensive selective serotonin reuptake inhibitor
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.
SSRIs increase the extracellul ...
(SSRI) antidepressant class and is the best-selling antidepressant. Many other SSRI antidepressants are fluorinated organics, including Celexa
Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and soci ...
, Luvox, and Lexapro. Fluoroquinolones
A quinolone antibiotic is a member of a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as we ...
are a commonly used family of broad-spectrum antibiotic
A broad-spectrum antibiotic is an antibiotic that acts on the two major bacterial groups, Gram-positive and Gram-negative, or any antibiotic that acts against a wide range of disease-causing bacteria. These medications are used when a bacterial ...
s.
Scanning
radiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which ...
is 2-deoxy-2-(18F)fluoro-D-glucose (generically referred to as fludeoxyglucose), commonly abbreviated as 18F-FDG, or simply FDG. In PET imaging, FDG can be used for assessing glucose metabolism in the brain and for imaging cancer tumors. After injection into the blood, FDG is taken up by "FDG-avid" tissues with a high need for glucose, such as the brain and most types of malignant tumors. Tomography
Tomography is imaging by sections or sectioning that uses any kind of penetrating wave. The method is used in radiology, archaeology, biology, atmospheric science, geophysics, oceanography, plasma physics, materials science, astrophysics, ...
, often assisted by a computer to form a PET/CT (CT stands for "computer tomography") machine, can then be used to diagnose or monitor treatment of cancers, especially Hodgkin's lymphoma
Hodgkin lymphoma (HL) is a type of lymphoma, in which cancer originates from a specific type of white blood cell called lymphocytes, where multinucleated Reed–Sternberg cells (RS cells) are present in the patient's lymph nodes. The condition w ...
, lung cancer, and breast cancer.
Natural fluorine is monoisotopic, consisting solely of fluorine-19
Fluorine (9F) has 18 known isotopes ranging from to (with the exception of ) and two isomers ( and ). Only fluorine-19 is stable and naturally occurring in more than trace quantities; therefore, fluorine is a monoisotopic and mononuclidic elem ...
. Fluorine compounds are highly amenable to nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
(NMR), because fluorine-19 has a nuclear spin of , a high nuclear magnetic moment
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is mainly a magnetic dipole moment; the quadrupole moment does cause some small shifts in the hyperfine structure as ...
, and a high magnetogyric ratio
In physics, the gyromagnetic ratio (also sometimes known as the magnetogyric ratio in other disciplines) of a particle or system is the ratio of its magnetic moment to its angular momentum, and it is often denoted by the symbol Gamma, , gamma. Its ...
. Fluorine compounds typically have a fast NMR relaxation, which enables the use of fast averaging to obtain a signal-to-noise ratio similar to hydrogen-1
Hydrogen (1H) has three naturally occurring isotopes, sometimes denoted , , and . and are stable, while has a half-life of years. Heavier isotopes also exist, all of which are synthetic and have a half-life of less than one zeptosecond (10� ...
NMR spectra. Fluorine-19 is commonly used in NMR study of metabolism, protein structures and conformational changes. In addition, inert fluorinated gases have the potential to be a cheap and efficient tool for imaging lung ventilation.
Oxygen transport research
Liquid fluorocarbons have a very high capacity for holding gas in solution. They can hold more oxygen or carbon dioxide than blood does. For that reason, they have attracted ongoing interest related to the possibility of artificial blood or of liquid breathing. Blood substitutes are the subject of research because the demand for blood transfusions grows faster than donations. In some scenarios, artificial blood may be more convenient or safe. Because fluorocarbons do not normally mix with water, they must be mixed into emulsions (small droplets of perfluorocarbon suspended in water) in order to be used as blood. One such product, Oxycyte, has been through initial clinical trials.
Possible medical uses of liquid breathing (which uses pure perfluorocarbon liquid, not a water emulsion) involve assistance for premature babies or for burn patients (if normal lung function is compromised). Both partial and complete filling of the lungs have been considered, although only the former has undergone any significant tests in humans. Several animal tests have been performed and there have been some human partial liquid ventilation trials. One effort, by Alliance Pharmaceuticals, reached clinical trials but was abandoned because of insufficient advantage compared to other therapies.
Nanocrystals represent a possible method of delivering water- or fat-soluble drugs within a perfluorochemical fluid. The use of these particles is being developed to help treat babies with damaged lungs.
Perfluorocarbons are banned from sports, where they may be used to increase oxygen use for endurance athletes. One cyclist, Mauro Gianetti, was investigated after a near-fatality where PFC use was suspected. Other posited applications include deep-sea diving and space travel, applications that both require total, not partial, liquid ventilation. The 1989 film ''
Blood substitutes are the subject of research because the demand for blood transfusions grows faster than donations. In some scenarios, artificial blood may be more convenient or safe. Because fluorocarbons do not normally mix with water, they must be mixed into emulsions (small droplets of perfluorocarbon suspended in water) in order to be used as blood. One such product, Oxycyte, has been through initial clinical trials.
Possible medical uses of liquid breathing (which uses pure perfluorocarbon liquid, not a water emulsion) involve assistance for premature babies or for burn patients (if normal lung function is compromised). Both partial and complete filling of the lungs have been considered, although only the former has undergone any significant tests in humans. Several animal tests have been performed and there have been some human partial liquid ventilation trials. One effort, by Alliance Pharmaceuticals, reached clinical trials but was abandoned because of insufficient advantage compared to other therapies.
Nanocrystals represent a possible method of delivering water- or fat-soluble drugs within a perfluorochemical fluid. The use of these particles is being developed to help treat babies with damaged lungs.
Perfluorocarbons are banned from sports, where they may be used to increase oxygen use for endurance athletes. One cyclist, Mauro Gianetti, was investigated after a near-fatality where PFC use was suspected. Other posited applications include deep-sea diving and space travel, applications that both require total, not partial, liquid ventilation. The 1989 film ''The Abyss
''The Abyss'' is a 1989 American science fiction film written and directed by James Cameron and starring Ed Harris, Mary Elizabeth Mastrantonio, and Michael Biehn. When an American submarine sinks in the Caribbean, a US search and recovery tea ...
'' depicted a fictional use of perfluorocarbon for human diving but also filmed a real rat surviving while cooled and immersed in perfluorocarbon. (See also list of fictional treatments of perfluorocarbon breathing.)
Agrichemicals
An estimated 30% of agrichemical compounds contain fluorine. Most of them are used as poisons, but a few stimulate growth instead.Sodium fluoroacetate
Sodium fluoroacetate is an organofluorine chemical compound with the formula FCH2CO2Na. This colourless salt has a taste similar to that of sodium chloride and is used as a rodenticide.
History and production
The effectiveness of sodium fluoroa ...
has been used as an insecticide, but it is especially effective against mammalian pests. The name "1080" refers to the catalogue number of the poison, which became its brand name. Fluoroacetate is similar to acetate, which has a pivotal role in the Krebs cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
(a key part of cell metabolism). Fluoroacetate halts the cycle and causes cells to be deprived of energy. Several other insecticides contain sodium fluoride, which is much less toxic than fluoroacetate. Insects fed 29-fluorostigmasterol use it to produce fluoroacetates. If a fluorine is transferred to a body cell, it blocks metabolism at the position occupied.
Trifluralin was widely used in the 20th century, for example, in over half of U.S. cotton field acreage in 1998. Because of its suspected carcinogenic properties some Northern European countries banned it in 1993. As of 2015, the European Union has banned it, although Dow made a case to cancel the decision in 2011.
Biochemistry
 Biologically synthesized organofluorines are few in number, although some are widely produced. The most common example is
Biologically synthesized organofluorines are few in number, although some are widely produced. The most common example is fluoroacetate
Fluoroacetate may refer to:
* Fluoroacetic acid
* Sodium fluoroacetate
{{Short pages monitor