Barium ferrate on:
[Wikipedia]
[Google]
[Amazon]
Barium ferrate is the /nowiki>FeO4 2− anion.

 BaFeO4 follows the
BaFeO4 follows the
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
of formula BaFeO4. This is a rare compound containing iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
in the +6 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. It is isostructural with BaSO4, and contains the tetrahedral Structure
The ferrate(VI) anion is paramagnetic due to its twounpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain ...
s and it has a tetrahedral molecular geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccosine, cos−1(−) = 109.4712206...° ≈ 109.5° when all four substit ...
.
X-ray diffraction has been used to determine the orthorhombic unit cell structure (lattice vectors a ≠ b ≠ c, interaxial angles α=β=γ=90°) of nanocrystalline BaFeO4. It crystallized in the Pnma space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it uncha ...
(point group: D2h) with lattice parameters ''a'' = 0.8880 nm, ''b'' = 0.5512 nm and ''c'' = 0.7214 nm. The accuracy of the X-Ray diffraction data has been verified by the lattice fringe intervals from High-Resolution Transmission Electron Microscopy
High-resolution transmission electron microscopy is an imaging mode of specialized transmission electron microscopes that allows for direct imaging of the atomic structure of samples. It is a powerful tool to study properties of materials on the a ...
(HRTEM) and cell parameters calculated from Selected Area Diffraction (SAED).

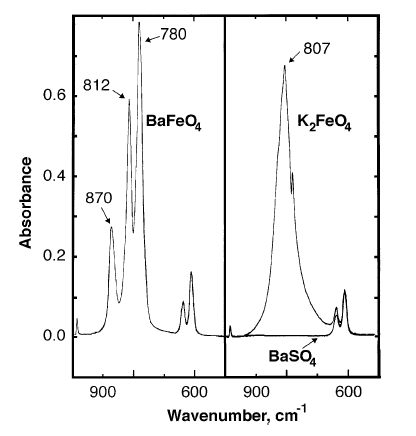
Characterization
Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
absorbance peaks of barium ferrate are observed at 870, 812, 780 cm−1.
 BaFeO4 follows the
BaFeO4 follows the Curie–Weiss law
The Curie–Weiss law describes the magnetic susceptibility of a ferromagnet in the paramagnetic region above the Curie point:
:
\chi = \frac
where is a material-specific Curie constant, is the absolute temperature, and is the Curie tempera ...
and has a magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagne ...
of (2.92 ± 0.03) × 10−23 A m2 (3.45 ± 0.1 BM) with a Weiss constant
Weiss or Weiß may refer to:
People
* Weiss (surname), including spelling Weiß
* Weiss Ferdl (1883-1949), German actor
Places
* Mount Weiss, Jasper National Park, Alberta, Canada
* Weiss Lake, Alabama
* Weiß (Sieg), a river in North Rhine-Westp ...
of −89 K.
Preparation and chemistry
Barium ferrate(VI) can be prepared by both wet and dry synthetic methods. Dry synthesis is usually performed using a thermal technique, such as by heatingbarium hydroxide
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (''x'' = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.
...
and iron(II) hydroxide in the presence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
to about 800 to 900 °C.
: + + → + 2
Wet methods employ both chemical and electrochemical techniques. For example, the ferrate anion forms when a suitable iron salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
is placed in alkaline conditions and a strong oxidising agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
, such as sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium s ...
, is added.
:2 + 3 + 4 → 2 + 5 + 3
Barium ferrate is then precipitated from solution by adding a solution of a barium(II) salt. Addition of a soluble barium salt to an alkali metal ferrate solution produces a maroon precipitate of barium ferrate, a crystal which has the same structure as barium chromate
Barium chromate, named barium tetraoxochromate(VI) by the IUPAC, is a yellow sand like powder with the formula BaCrO4. It is a known oxidizing agent and produces a green flame when heated, a result of the barium ions.
History
The first naturally o ...
and has approximately the same solubility. Barium ferrate has also been prepared by adding barium oxide
Barium oxide, also known as baria, is a white hygroscopic non-flammable compound with the formula BaO. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and if swallowed in larg ...
to a mixture sodium hypochlorite and ferric nitrate at room temperature (or 0 °C). The purity of the product can be improved by carrying out the reaction at low temperature in the absence of carbon dioxide and by rapidly filtering and drying the precipitate, reducing the coprecipitation of barium hydroxide
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (''x'' = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.
...
and barium carbonate
Barium carbonate is the inorganic compound with the formula BaCO3. Like most alkaline earth metal carbonates, it is a white salt that is poorly soluble in water. It occurs as the mineral known as witherite. In a commercial sense, it is one of ...
as impurities.
Uses
Barium ferrate is anoxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
and is used as an oxidizing reagent in organic syntheses. Its other applications include removal of color, removal of cyanide, killing bacteria and contaminated and waste water treatment.
Salts of ferrate(VI) are energetic cathode materials in "super-iron" batteries. Cathodes containing ferrate(VI) compounds are referred to as "super-iron" cathodes due to their highly oxidized iron basis, multiple electron transfer, and high intrinsic energy. Among all ferrate(VI) salts, barium ferrate sustains unusually facile charge transfer, which is important for the high power domain of alkaline batteries
An alkaline battery (IEC code: L) is a type of primary battery where the electrolyte (most commonly potassium hydroxide) has a pH value above 7. Typically these batteries derive energy from the reaction between zinc metal and manganese dioxi ...
.
Reactions
Barium ferrate is the most stable of the ferrate(VI) compounds. It can be prepared in its purest state and has the most definite composition. Barium ferrate can be easily decomposed by all soluble acids, including carbonic acid. If carbon dioxide is passed through water on which hydrated barium ferrate is suspended, barium ferrate will decompose completely to formbarium carbonate
Barium carbonate is the inorganic compound with the formula BaCO3. Like most alkaline earth metal carbonates, it is a white salt that is poorly soluble in water. It occurs as the mineral known as witherite. In a commercial sense, it is one of ...
, ferric hydroxide and oxygen gas. Alkaline sulfates decompose barium ferrate that has not been dried, forming barium sulfate, ferric hydroxide and oxygen gas.
See also
* Potassium ferrate * Ferrate(VI) * BariumReferences
{{iron compounds Barium compounds Ferrates iron compounds