Baeyer–Villiger oxidation on:
[Wikipedia]
[Google]
[Amazon]
The Baeyer–Villiger oxidation is an organic reaction that forms an 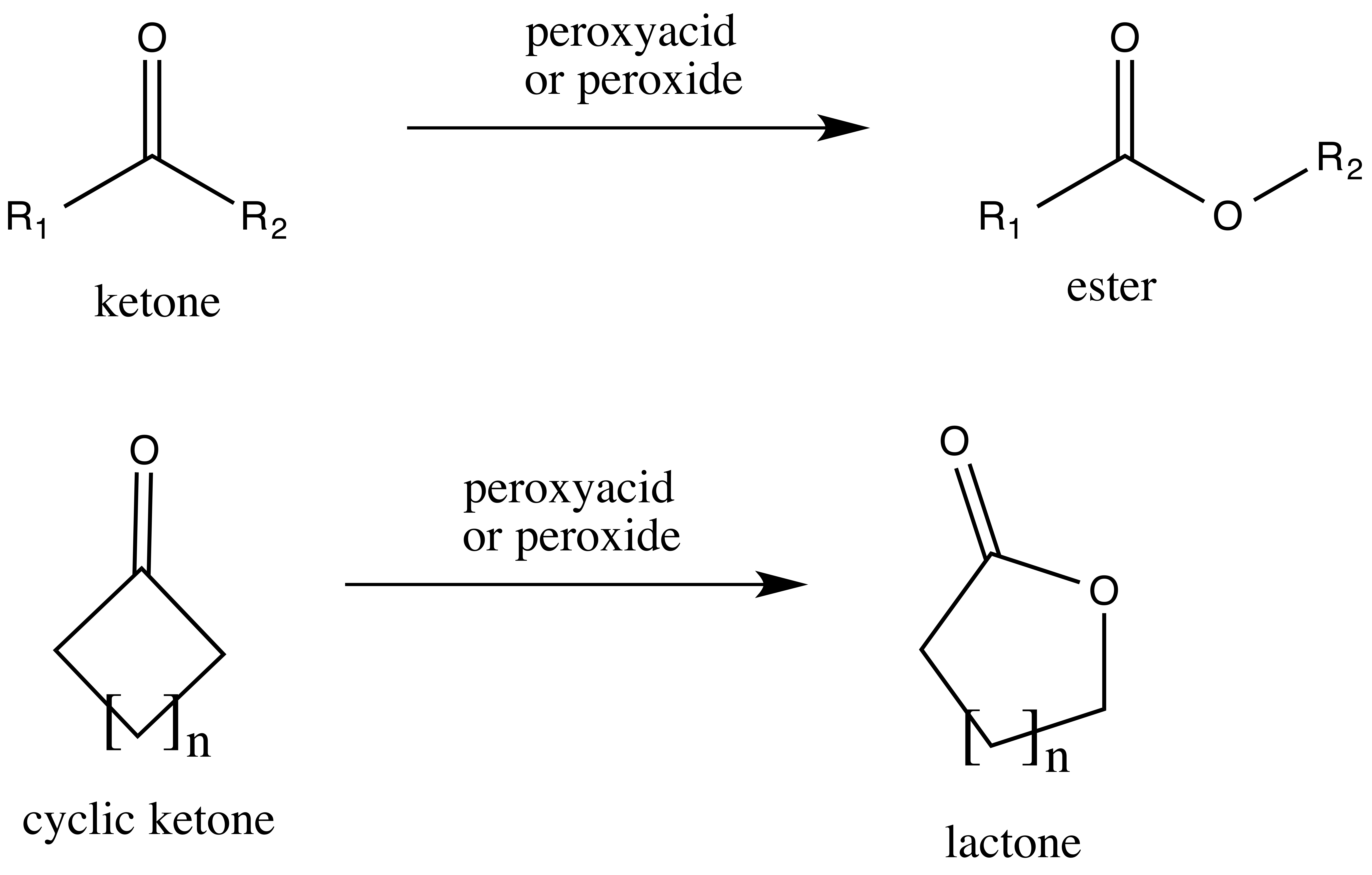
 The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and secondary
The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and secondary  The migratory ability is ranked tertiary > secondary > aryl > primary. Allylic groups are more apt to migrate than primary alkyl groups but less so than secondary alkyl groups. Electron-withdrawing groups on the substituent decrease the rate of migration. There are two explanations for this trend in migration ability. One explanation relies on the buildup of positive charge in the transition state for breakdown of the Criegee intermediate (illustrated by the carbocation
The migratory ability is ranked tertiary > secondary > aryl > primary. Allylic groups are more apt to migrate than primary alkyl groups but less so than secondary alkyl groups. Electron-withdrawing groups on the substituent decrease the rate of migration. There are two explanations for this trend in migration ability. One explanation relies on the buildup of positive charge in the transition state for breakdown of the Criegee intermediate (illustrated by the carbocation  Another explanation uses stereoelectronic effects and steric arguments. As mentioned, the substituent that is antiperiplanar to the peroxide group in the
Another explanation uses stereoelectronic effects and steric arguments. As mentioned, the substituent that is antiperiplanar to the peroxide group in the  The migrating group in acyclic ketones, usually, is not 1° alkyl group. However, they may be persuaded to migrate in preference to the 2° or 3° groups by using CF3CO3H or BF3 + H2O2 as reagents.
The migrating group in acyclic ketones, usually, is not 1° alkyl group. However, they may be persuaded to migrate in preference to the 2° or 3° groups by using CF3CO3H or BF3 + H2O2 as reagents.
 There were three suggested reaction mechanisms of the Baeyer–Villiger oxidation that seemed to fit with observed reaction outcomes. These three reaction mechanisms can really be split into two pathways of peroxyacid attack – on either the oxygen or the carbon of the
There were three suggested reaction mechanisms of the Baeyer–Villiger oxidation that seemed to fit with observed reaction outcomes. These three reaction mechanisms can really be split into two pathways of peroxyacid attack – on either the oxygen or the carbon of the  In 1953,
In 1953, 

 In nature,
In nature,


Animation of the Baeyer–Villiger oxidation
{{DEFAULTSORT:Baeyer-Villiger Oxidation Organic oxidation reactions Esterification reactions Name reactions
ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
from a ketone or a lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
from a cyclic ketone, using peroxyacids or peroxides
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
as the oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
. The reaction is named after Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo and developed a nomenclature for cyclic compounds (that was subsequently extended and adopted as part of the IUPAC org ...
and Victor Villiger who first reported the reaction in 1899.

Reaction mechanism
In the first step of the reaction mechanism, the peroxyacid protonates the oxygen of thecarbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
. This makes the carbonyl group more susceptible to be attacked by the peroxyacid. Next, the peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate
A Criegee intermediate (also called a Criegee zwitterion or Criegee biradical) is a carbonyl oxide with two charge centres. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the f ...
. Through a concerted mechanism, one of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
. Finally, deprotonation of the oxocarbenium ion produces the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
.
 The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and secondary
The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and secondary stereoelectronic effect
In chemistry, primarily organic and computational chemistry, a stereoelectronic effectAlabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu.wiley.com/WileyCDA/W ...
s. The primary stereoelectronic effect in the Baeyer–Villiger oxidation refers to the necessity of the oxygen-oxygen bond in the peroxide group to be antiperiplanar to the group that migrates. This orientation facilitates optimum overlap of the 𝛔 orbital of the migrating group to the 𝛔* orbital of the peroxide group. The secondary stereoelectronic effect refers to the necessity of the lone pair on the oxygen of the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
group to be antiperiplanar to the migrating group. This allows for optimum overlap of the oxygen nonbonding orbital with the 𝛔* orbital of the migrating group. This migration step is also (at least '' in silico'') assisted by two or three peroxyacid units enabling the hydroxyl proton to shuttle to its new position.
 The migratory ability is ranked tertiary > secondary > aryl > primary. Allylic groups are more apt to migrate than primary alkyl groups but less so than secondary alkyl groups. Electron-withdrawing groups on the substituent decrease the rate of migration. There are two explanations for this trend in migration ability. One explanation relies on the buildup of positive charge in the transition state for breakdown of the Criegee intermediate (illustrated by the carbocation
The migratory ability is ranked tertiary > secondary > aryl > primary. Allylic groups are more apt to migrate than primary alkyl groups but less so than secondary alkyl groups. Electron-withdrawing groups on the substituent decrease the rate of migration. There are two explanations for this trend in migration ability. One explanation relies on the buildup of positive charge in the transition state for breakdown of the Criegee intermediate (illustrated by the carbocation resonance structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
of the Criegee intermediate). Keeping this structure in mind, it makes sense that the substituent that can maintain positive charge the best would be most likely to migrate. The higher the degree of substitution, the more stable a carbocation generally is. Therefore, the tertiary > secondary > primary trend is observed.
 Another explanation uses stereoelectronic effects and steric arguments. As mentioned, the substituent that is antiperiplanar to the peroxide group in the
Another explanation uses stereoelectronic effects and steric arguments. As mentioned, the substituent that is antiperiplanar to the peroxide group in the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
will migrate. This transition state has a gauche interaction between the peroxyacid and the non-migrating substituent. If the bulkier group is placed antiperiplanar to the peroxide group, the gauche interaction between the substituent on the forming ester and the carbonyl group of the peroxyacid will be reduced. Thus, it is the bulkier group that will prefer to be antiperiplanar to the peroxide group, enhancing its aptitude for migration.
 The migrating group in acyclic ketones, usually, is not 1° alkyl group. However, they may be persuaded to migrate in preference to the 2° or 3° groups by using CF3CO3H or BF3 + H2O2 as reagents.
The migrating group in acyclic ketones, usually, is not 1° alkyl group. However, they may be persuaded to migrate in preference to the 2° or 3° groups by using CF3CO3H or BF3 + H2O2 as reagents.
Historical background
In 1899, Adolf Baeyer and Victor Villiger first published a demonstration of the reaction that we now know as the Baeyer–Villiger oxidation. They used peroxymonosulfuric acid to make the corresponding lactones from camphor,menthone
Menthone is a monoterpene with a minty flavor that occurs naturally in a number of essential oils. ''l''-Menthone (or (2''S'',5''R'')-''trans''-2-isopropyl-5-methylcyclohexanone), shown at right, is the most abundant in nature of the four possible ...
, and tetrahydrocarvone.
 There were three suggested reaction mechanisms of the Baeyer–Villiger oxidation that seemed to fit with observed reaction outcomes. These three reaction mechanisms can really be split into two pathways of peroxyacid attack – on either the oxygen or the carbon of the
There were three suggested reaction mechanisms of the Baeyer–Villiger oxidation that seemed to fit with observed reaction outcomes. These three reaction mechanisms can really be split into two pathways of peroxyacid attack – on either the oxygen or the carbon of the carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
. Attack on oxygen could lead to two possible intermediates: Baeyer and Villiger suggested a dioxirane
In chemistry, dioxirane is a compound with formula , whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.
Th ...
intermediate, while Georg Wittig
Georg Wittig (; 16 June 1897 – 26 August 1987) was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Che ...
and Gustav Pieper suggested a peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
with no dioxirane formation. Carbon attack was suggested by Rudolf Criegee. In this pathway, the peracid attacks the carbonyl carbon, producing what is now known as the Criegee intermediate
A Criegee intermediate (also called a Criegee zwitterion or Criegee biradical) is a carbonyl oxide with two charge centres. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the f ...
.
 In 1953,
In 1953, William von Eggers Doering
William von Eggers Doering (June 22, 1917 – January 3, 2011) was the Mallinckrodt Professor of Chemistry at Harvard University. Before Harvard, he taught at Columbia (1942–1952) and Yale (1952–1968).
Doering was born in Fort Worth, Texa ...
and Edwin Dorfman elucidated the correct pathway for the reaction mechanism of the Baeyer–Villiger oxidation by using oxygen-18
Oxygen-18 (, Ω) is a natural, stable isotope of oxygen and one of the environmental isotopes.
is an important precursor for the production of fluorodeoxyglucose (FDG) used in positron emission tomography (PET). Generally, in the radiopharmaceu ...
-labelling of benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylket ...
. The three different mechanisms would each lead to a different distribution of labelled products. The Criegee intermediate would lead to a product only labelled on the carbonyl oxygen. The product of the Wittig and Pieper intermediate is only labeled on the alkoxy group
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also k ...
of the ester. The Baeyer and Villiger intermediate leads to a 1:1 distribution of both of the above products. The outcome of the labelling experiment supported the Criegee intermediate, which is now the generally accepted pathway.

Stereochemistry
The migration does not change the stereochemistry of the group that transfers, i.e.: it is stereoretentive.Reagents
Although many different peroxyacids are used for the Baeyer–Villiger oxidation, some of the more commonoxidants
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
include ''meta''-chloroperbenzoic acid (mCPBA) and trifluoroperacetic acid
Trifluoroperacetic acid (trifluoroperoxyacetic acid, TFPAA) is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula . It is a strong oxidizing agent for organic oxidation reactions, such ...
(TFPAA). The general trend is that higher reactivity is correlated with lower pKa (i.e.: stronger acidity) of the corresponding carboxylic acid (or alcohol in the case of the peroxides). Therefore, the reactivity trend shows TFPAA > 4-nitroperbenzoic acid > mCPBA and performic acid
Performic acid (PFA) is an organic compound with the formula CH2O3. It is an unstable colorless liquid which can be produced by mixing formic acid with hydrogen peroxide. Owing to its oxidizing and disinfecting action, it is used in the chemical, ...
> peracetic acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the chemical formula, formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corros ...
> hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
> tert-butyl hydroperoxide
''tert''-Butyl hydroperoxide (tBuOOH) is the organic compound with the formula (CH3)3COOH. It is one of the most widely used hydroperoxides in a variety of oxidation processes, for example the Halcon process. It is normally supplied as a 69– ...
. The peroxides are much less reactive than the peroxyacids. The use of hydrogen peroxide even requires a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. In addition, using organic peroxides and hydrogen peroxide tends to generate more side-reactivity due to their promiscuity.
Limitations
The use of peroxyacids andperoxides
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
when performing the Baeyer–Villiger oxidation can cause the undesirable oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of other functional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
. Alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
and amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
are a few of the groups that can be oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
. For instance, alkenes in the substrate, particularly when electron-rich, may be oxidized to epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
s. However, methods have been developed that will allow for the tolerance of these functional groups. In 1962, G. B. Payne reported that the use of hydrogen peroxide in the presence of a selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
catalyst will produce the epoxide from alkenyl ketones, while use of peroxyacetic acid will form the ester.

Modifications
Catalytic Baeyer-Villiger oxidation
The use of hydrogen peroxide as anoxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
would be advantageous, making the reaction more environmentally friendly as the sole byproduct is water. Benzeneseleninic acid derivatives as catalysts have been reported to give high selectivity with hydrogen peroxide as the oxidant. Another class of catalysts which show high selectivity with hydrogen peroxide as the oxidant are solid Lewis acid catalysts such as stannosilicates. Among stannosilicates, particularly the zeotype Sn-beta and the amorphous Sn-MCM-41 show promising activity and close to full selectivity towards the desired product.
Asymmetric Baeyer-Villiger oxidation
There have been attempts to use organometallic catalysts to perform enantioselective Baeyer–Villiger oxidations. The first reported instance of one such oxidation of a prochiral ketone used dioxygen as the oxidant with a copper catalyst. Other catalysts, including platinum and aluminum compounds, followed.Baeyer-Villiger monooxygenases
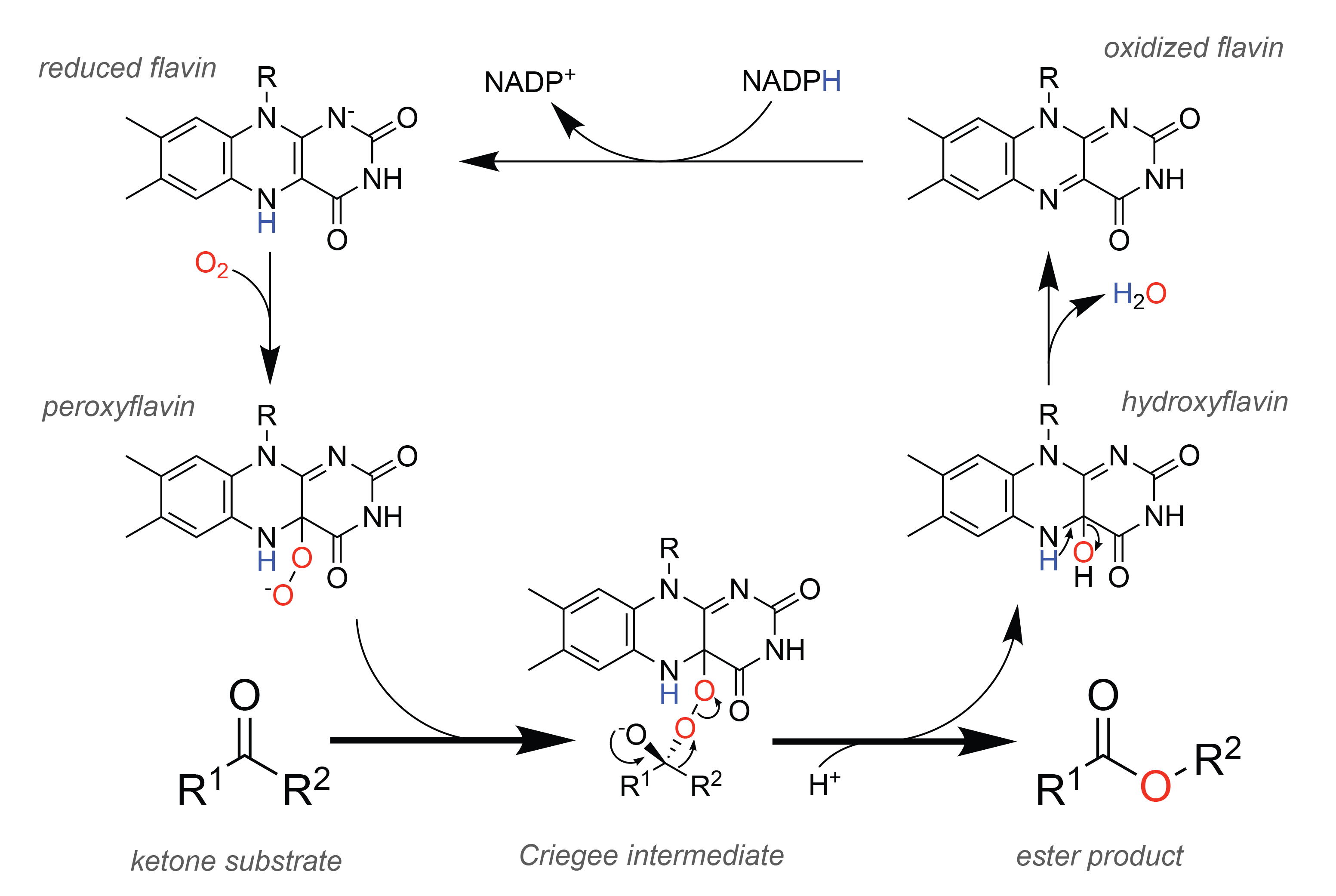
 In nature,
In nature, enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s called Baeyer-Villiger monooxygenases (BVMOs) perform the oxidation analogously to the chemical reaction. To facilitate this chemistry, BVMOs contain a flavin adenine dinucleotide (FAD) cofactor. In the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
(see figure on the right), the cellular redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
equivalent NADPH first reduces the cofactor, which allows it subsequently to react with molecular oxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
* ...
. The resulting peroxyflavin is the catalytic entity oxygenating the substrate, and theoretical studies suggest that the reaction proceeds through the same Criegee intermediate as observed in the chemical reaction. After the rearrangement step forming the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
product, a hydroxyflavin remains, which spontaneously eliminates water to form oxidized flavin, thereby closing the catalytic cycle.
BVMOs are closely related to the flavin-containing monooxygenase
The flavin-containing monooxygenase (FMO) protein family specializes in the oxidation of xeno-substrates in order to facilitate the excretion of these compounds from living organisms. These enzymes can oxidize a wide array of heteroatoms, par ...
s (FMOs), enzymes that also occur in the human body, functioning within the frontline metabolic detoxification
Detoxification or detoxication (detox for short) is the physiological or medicinal removal of toxic substances from a living organism, including the human body, which is mainly carried out by the liver. Additionally, it can refer to the period of ...
system of the liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
along the cytochrome P450 monooxygenases. Human FMO5 was in fact shown to be able to catalyse Baeyer-Villiger reactions, indicating that the reaction may occur in the human body as well.
BVMOs have been widely studied due to their potential as biocatalysts, that is, for an application in organic synthesis. Considering the environmental concerns for most of the chemical catalysts, the use of enzymes is considered a greener alternative. BVMOs in particular are interesting for application because they fulfil a range of criteria typically sought for in biocatalysis: besides their ability to catalyse a synthetically useful reaction, some natural homologs
A couple of homologous chromosomes, or homologs, are a set of one maternal and one paternal chromosome that pair up with each other inside a cell during fertilization. Homologs have the same genes in the same loci where they provide points alon ...
were found to have a very large substrate scope (i.e. their reactivity was not restricted to a single compound, as often assumed in enzyme catalysis), they can be easily produced on a large scale, and because the three-dimensional structure of many BVMOs has been determined, enzyme engineering could be applied to produce variants with improved thermostability
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature.
...
and/or reactivity. Another advantage of using enzymes for the reaction is their frequently observed regio- and enantioselectivity, owed to the steric control of substrate orientation during catalysis within the enzyme's active site.
Applications
Zoapatanol
Zoapatanol is a biologically active molecule that occurs naturally in the zeopatle plant, which has been used in Mexico to make a tea that can induce menstruation and labor. In 1981, Vinayak Kane and Donald Doyle reported a synthesis of zoapatanol. They used the Baeyer–Villiger oxidation to make alactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
that served as a crucial building block that ultimately led to the synthesis of zoapatanol.

Steroids
In 2013, Alina Świzdor reported the transformation of the steroiddehydroepiandrosterone
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It fun ...
to anticancer agent testololactone by use of a Baeyer–Villiger oxidation induced by fungus that produces Baeyer-Villiger monooxygenases.

See also
*Dakin reaction
The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an '' ortho''- or ''para''-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide in base to form a ...
References
External links
Animation of the Baeyer–Villiger oxidation
{{DEFAULTSORT:Baeyer-Villiger Oxidation Organic oxidation reactions Esterification reactions Name reactions