Bacteriorhodopsin on:
[Wikipedia]
[Google]
[Amazon]
Bacteriorhodopsin is a protein used by Archaea, most notably by
 Bacteriorhodopsin is a 27
Bacteriorhodopsin is a 27
 The bacteriorhodopsin photocycle consists of nine distinct stages, starting from the ground or resting state, which is denoted 'bR'. The intermediates are identified by single letters and may be distinguished by their
The bacteriorhodopsin photocycle consists of nine distinct stages, starting from the ground or resting state, which is denoted 'bR'. The intermediates are identified by single letters and may be distinguished by their
File:Bacteriorhodopsin subunit 1X0S.gif, link=File:Bacteriorhodopsin subunit 1X0S large.gif, Bacteriorhodopsin single
RasTop
(Molecular Visualization Software).). One more small helix is light blue, beta sheet yellow. File:Bacteriorhodopsin trimer 1X0S.png, link=File:Bacteriorhodopsin trimer 1X0S large.gif, Bacteriorhodopsin trimer with one
Bacteriorhodopsin: Molecule of the Month
by David Goodsell, RCSB Protein Data Bank
by Nicole Wagner & Jordan Greco {{Proton pumps 7TM receptors Photosynthesis Integral membrane proteins Archaea proteins
haloarchaea
Haloarchaea (halophilic archaea, halophilic archaebacteria, halobacteria) are a class of the Euryarchaeota, found in water saturated or nearly saturated with salt. Halobacteria are now recognized as archaea rather than bacteria and are one of t ...
, a class
Class or The Class may refer to:
Common uses not otherwise categorized
* Class (biology), a taxonomic rank
* Class (knowledge representation), a collection of individuals or objects
* Class (philosophy), an analytical concept used differentl ...
of the Euryarchaeota
Euryarchaeota (from Ancient Greek ''εὐρύς'' eurús, "broad, wide") is a phylum of archaea. Euryarchaeota are highly diverse and include methanogens, which produce methane and are often found in intestines, halobacteria, which survive extr ...
. It acts as a proton pump
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell f ...
; that is, it captures light energy and uses it to move protons across the membrane out of the cell. The resulting proton gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and th ...
is subsequently converted into chemical energy.
Function
Bacteriorhodopsin is a light-driven H+ ion transporter found in some haloarchaea, most notably '' Halobacterium salinarum'' (formerly known as syn. ''H. halobium''). Theproton-motive force
Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membra ...
generated by the protein is used by ATP synthase to generate adenosine triphosphate (ATP). By expressing bacteriorhodopsin, the archaea cells are able to synthesise ATP in the absence of a carbon source.
Structure
 Bacteriorhodopsin is a 27
Bacteriorhodopsin is a 27 kDa
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at re ...
integral membrane protein
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All ''transmembrane proteins'' are IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a sign ...
usually found in two-dimensional crystalline patches known as "purple membrane", which can occupy almost 50% of the surface area of the archaeal cell. The repeating element of the hexagonal lattice is composed of three identical protein chains, each rotated by 120 degrees relative to the others. Each monomer has seven transmembrane alpha helices and an extracellular-facing, two-stranded beta sheet.
Bacteriorhodopsin is synthesized as a protein precursor
A protein precursor, also called a pro-protein or pro-peptide, is an inactive protein (or peptide) that can be turned into an active form by post-translational modification, such as breaking off a piece of the molecule or adding on another molecule ...
, known as bacterio-opsin, which is extensively modified after translation. The modifications are:
* Covalent conjugation of a retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use reti ...
molecule to residue Lys216, via a Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
, to create the retinylidene chromophore.
* Cleavage of the signal peptide, the first 13 amino acids at the N-terminus, and the conversion of residue Gln14 to pyroglutamate
* Removal of residue Asp262 at the C-terminus
Spectral properties
Bacteriorhodopsin molecule is purple and is most efficient at absorbing green light (in the wavelength range 500-650 nm). In the native membrane, the protein has a maximum absorbance at 553 nm, however addition of detergent disrupts the trimeric form, leading a loss of exciton coupling between the chromophores, and the monomeric form consequently has an absorption maximum of 568 nm. Bacteriorhodopsin has a broad excitation spectrum. For a detection wavelength between 700 and 800 nm, it has an appreciable detected emission for excitation wavelengths between 470 nm and 650 nm (with a peak at 570 nm). When pumped at 633 nm, the emission spectrum has appreciable intensity between 650 nm and 850 nm.Mechanism
Photocycle overview
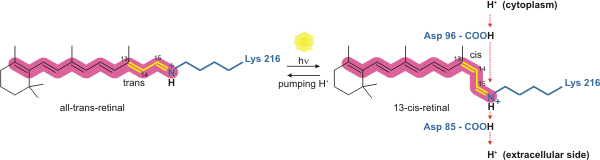
Bacteriorhodopsin is a light-driven proton pump. It is the retinal molecule that changes its isomerization state from all-''trans'' to 13-''cis'' when it absorbs aphoton
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
. The surrounding protein responds to the change in the chromophore shape, by undergoing an ordered sequence of conformational changes (collectively known as the photocycle). The conformational changes alter the p''K''a values of conserved amino acids in the core of the protein, including Asp85, Asp96 and the Schiff base N atom (Lys216). These sequential changes in acid dissociation constant, result in the transfer of one proton from the intracellular side to the extracellular side of the membrane for each photon absorbed by the chromophore.
 The bacteriorhodopsin photocycle consists of nine distinct stages, starting from the ground or resting state, which is denoted 'bR'. The intermediates are identified by single letters and may be distinguished by their
The bacteriorhodopsin photocycle consists of nine distinct stages, starting from the ground or resting state, which is denoted 'bR'. The intermediates are identified by single letters and may be distinguished by their absorption spectra
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating ...
. The nine stages are:
: bR + photon → K L M1 M2 M2' N N' O bR
Ground state + photon → K state → L state
Bacteriorhodopsin in the ground state absorbs a photon and the retinal changes isomerization from all-''trans'' 15-''anti'' to the strained 13-''cis'' 15-''anti'' in the K state. The isomerisation reaction is fast and occurs in less than 1 ps. The retinal adopts a less strained conformation to form the L intermediate.L state → M1 state
Asp85 accepts a proton from the Schiff base N atom. In the M1 intermediate, neither the Schiff base nor Asp85 are charged.M1 state → M2 state
The Schiff base rotates away from the extracelluar side of the protein towards the cytoplasmic side, in preparation to accept a new proton.M2 state → M2' state
A proton is released from Glu204 and Glu194 to the extracellular medium.M2' state → N state
The retinal Schiff base accepts a proton from Asp96. In the N state, both Asp96 and the Schiff base are charged.N state → N' state
Asp96 accepts a proton from the cytoplasmic side of the membrane and becomes uncharged.N' state → O state
Retinal reisomerizes to the all-''trans'' state.O state → ground state
Asp85 transfers a proton to Glu194 and Glu204 on the extracellular face of the protein.Homologs and other similar proteins
Bacteriorhodopsin belongs to themicrobial rhodopsin
Microbial rhodopsins, also known as bacterial rhodopsins are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic
and other bacteria. They are integral membrane proteins with seven transmembra ...
family. Its homologs include the archaerhodopsins, the light-driven chloride pump halorhodopsin Halorhodopsin is a light-gated ion pump, specific for chloride ions, found in archaea, known as halobacteria. It is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is similar in tertiary structure (but not primary se ...
(for which the crystal structure is also known), and some directly light-activated channels such as channelrhodopsin
Channelrhodopsins are a subfamily of retinylidene proteins ( rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Express ...
.
Bacteriorhodopsin is similar to vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () (chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, with c ...
rhodopsins, the pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s that sense light in the retina
The retina (from la, rete "net") is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then ...
. Rhodopsins also contain retinal; however, the functions of rhodopsin and bacteriorhodopsin are different, and there is limited similarity in their amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
sequences. Both rhodopsin and bacteriorhodopsin belong to the 7TM receptor family of proteins, but rhodopsin is a G protein-coupled receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related p ...
and bacteriorhodopsin is not. In the first use of electron crystallography
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope (TEM).
Comparison with X-ray crystallography
It can complement X-ray crystallography for studies of very small crystals ...
to obtain an atomic-level protein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monom ...
, the structure of bacteriorhodopsin was resolved in 1990. It was then used as a template to build models of G protein-coupled receptors before crystallographic structures were also available for these protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s. It has been excessively studied on both mica and glass substrates using Atomic force microscopy and Femtosecond crystallography.
All other phototroph
Phototrophs () are organisms that carry out photon capture to produce complex organic compounds (e.g. carbohydrates) and acquire energy. They use the energy from light to carry out various cellular metabolic processes. It is a common misconcep ...
ic systems in bacteria, algae, and plants use chlorophylls or bacteriochlorophyll
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by C. B. van Niel in 1932. They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanoba ...
s rather than bacteriorhodopsin. These also produce a proton gradient, but in a quite different and more indirect way involving an electron transfer chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples thi ...
consisting of several other proteins. Furthermore, chlorophylls are aided in capturing light energy by other pigments known as "antennas"; these are not present in bacteriorhodopsin-based systems. It is possible that phototrophy independently evolved at least twice, once in bacteria and once in archaea.
Gallery
monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
with retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use reti ...
molecule between 7 vertical alpha helixes ( PDB ID: 1X0S Image created witRasTop
(Molecular Visualization Software).). One more small helix is light blue, beta sheet yellow. File:Bacteriorhodopsin trimer 1X0S.png, link=File:Bacteriorhodopsin trimer 1X0S large.gif, Bacteriorhodopsin trimer with one
retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use reti ...
molecule in each subunit seen from the extracellular side EC ( PDB ID: 1X0S ).See also
*Microbial rhodopsin
Microbial rhodopsins, also known as bacterial rhodopsins are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic
and other bacteria. They are integral membrane proteins with seven transmembra ...
* Proteorhodopsin
Proteorhodopsin (also known as pRhodopsin) is a family of transmembrane proteins that use retinal as a chromophore for light-mediated functionality, in this case, a proton pump. pRhodopsin is found in marine planktonic bacteria, archaea and euka ...
* Opsin
Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become Retinylidene proteins, but are usually still called opsins regardless. Most ...
* Archaerhodopsin
* Purple Earth hypothesis
Literature
External links
Bacteriorhodopsin: Molecule of the Month
by David Goodsell, RCSB Protein Data Bank
by Nicole Wagner & Jordan Greco {{Proton pumps 7TM receptors Photosynthesis Integral membrane proteins Archaea proteins