Amide on:
[Wikipedia]
[Google]
[Amazon]


 In
In
 The lone pair of
The lone pair of  It is estimated that for acetamide, structure A makes a 62% contribution to the structure, while structure B makes a 28% contribution. (These figures do not sum to 100% because there are additional less-important resonance forms that are not depicted above). There is also a hydrogen bond present between the active groups hydrogen and nitrogen atoms. Resonance is largely prevented in the very strained quinuclidone.
It is estimated that for acetamide, structure A makes a 62% contribution to the structure, while structure B makes a 28% contribution. (These figures do not sum to 100% because there are additional less-important resonance forms that are not depicted above). There is also a hydrogen bond present between the active groups hydrogen and nitrogen atoms. Resonance is largely prevented in the very strained quinuclidone.
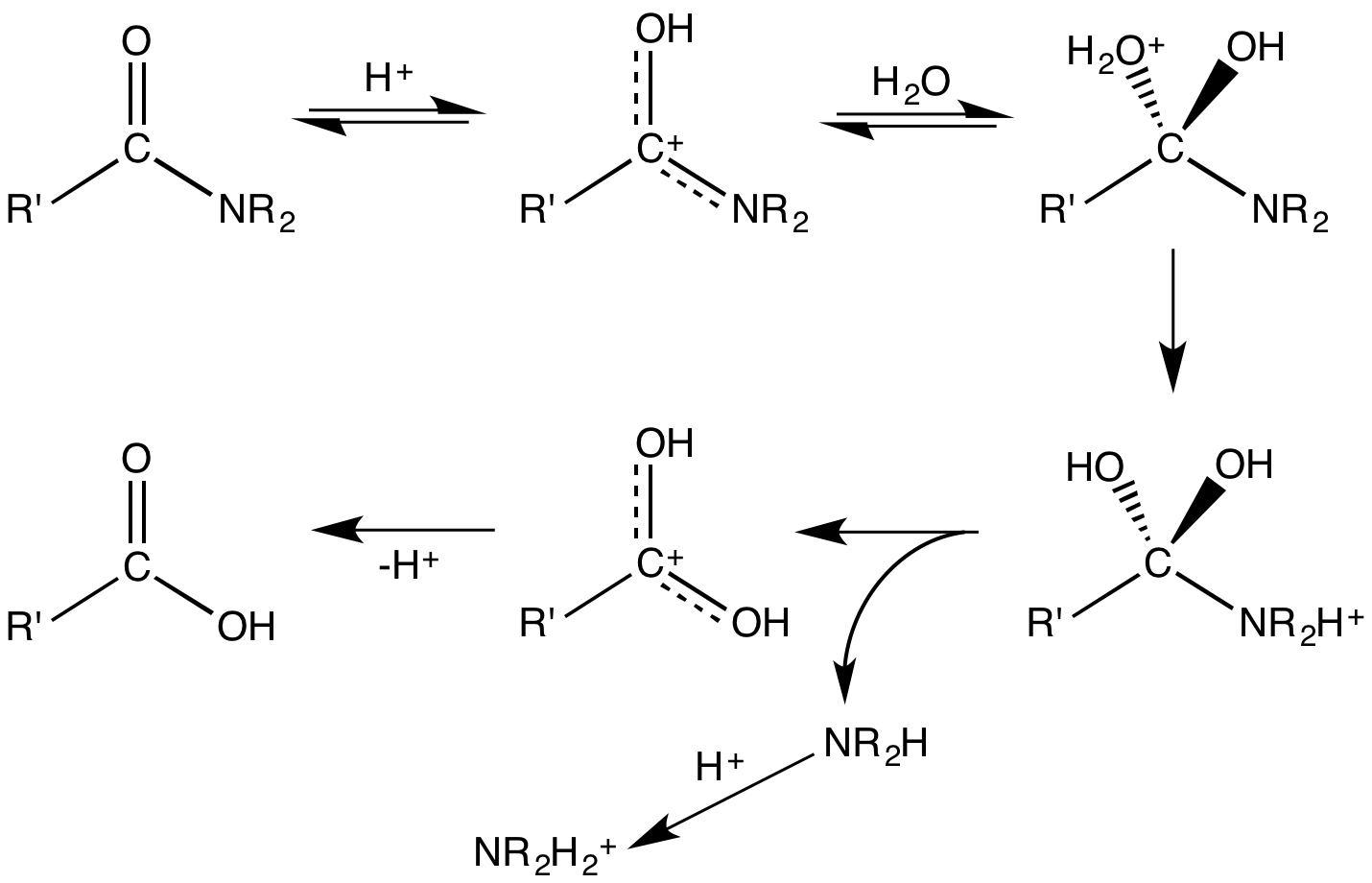 Amides undergo many chemical reactions, although they are less reactive than esters. Amides hydrolyse in hot alkali as well as in strong acidic conditions. Acidic conditions yield the carboxylic acid and the ammonium ion while basic hydrolysis yield the carboxylate ion and ammonia. The protonation of the initially generated amine under acidic conditions and the deprotonation of the initially generated carboxylic acid under basic conditions render these processes non-catalytic and irreversible. Amides are also versatile precursors to many other functional groups. Electrophiles react with the
Amides undergo many chemical reactions, although they are less reactive than esters. Amides hydrolyse in hot alkali as well as in strong acidic conditions. Acidic conditions yield the carboxylic acid and the ammonium ion while basic hydrolysis yield the carboxylate ion and ammonia. The protonation of the initially generated amine under acidic conditions and the deprotonation of the initially generated carboxylic acid under basic conditions render these processes non-catalytic and irreversible. Amides are also versatile precursors to many other functional groups. Electrophiles react with the
R^1 CO2H + R^2 R^3 NH -> R^2 R^3 NH2+R^1 CO2-
:R^2 R^3 NH2+R^1 CO2- -> R^1 C(O)NR^2 R^3 + H2O
Many methods involve "activating" the carboxylic acid by converting it to a better
 The reaction proceed by one dehydrogenation of the alcohol to the
The reaction proceed by one dehydrogenation of the alcohol to the RC(O)NR'_2 + HNR''_2 -> RC(O)NR''_2 + HNR'_2
Primary amides () are more amenable to this reaction.
IUPAC Compendium of Chemical Terminology
{{Authority control Functional groups

 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, an amide, also known as an organic amide or a carboxamide, is a compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
with the general formula , where R, R', and R″ represent organic groups or hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atoms. The amide group is called a peptide bond when it is part of the main chain
A ridge or a mountain ridge is a geographical feature consisting of a chain of mountains or hills that form a continuous elevated crest for an extended distance. The sides of the ridge slope away from the narrow top on either side. The line ...
of a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
, and an isopeptide bond when it occurs in a side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
, such as in the amino acids asparagine and glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
. It can be viewed as a derivative
In mathematics, the derivative of a function of a real variable measures the sensitivity to change of the function value (output value) with respect to a change in its argument (input value). Derivatives are a fundamental tool of calculus. ...
of a carboxylic acid () with the hydroxyl group () replaced by an amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group.
Common examples of amides are acetamide (), benzamide (), and dimethylformamide ().
Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen.
The core of amides is called the amide group (specifically, carboxamide group).
Amides are pervasive in nature and technology. Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s and important plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptab ...
s like Nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pe ...
s, Aramid, Twaron, and Kevlar
Kevlar (para-aramid) is a strong, heat-resistant synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, the high-strength material was first used commercially in the early 1970s a ...
are polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s whose units are connected by amide groups ( polyamides); these linkages are easily formed, confer structural rigidity, and resist hydrolysis. Amides include many other important biological compounds, as well as many drugs like paracetamol, penicillin and LSD
Lysergic acid diethylamide (LSD), also known colloquially as acid, is a potent psychedelic drug. Effects typically include intensified thoughts, emotions, and sensory perception. At sufficiently high dosages LSD manifests primarily mental, vi ...
. Low-molecular-weight amides, such as dimethylformamide, are common solvents.
Nomenclature
In the usual nomenclature, one adds the term "amide" to the stem of the parent acid's name. For instance, the amide derived from acetic acid is named acetamide (CH3CONH2). IUPAC recommendsethanamide
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound ''N'',''N''-dimeth ...
, but this and related formal names are rarely encountered. When the amide is derived from a primary or secondary amine, the substituents on nitrogen are indicated first in the name. Thus, the amide formed from dimethylamine and acetic acid is ''N'',''N''-dimethylacetamide (CH3CONMe2, where Me = CH3). Usually even this name is simplified to dimethylacetamide. Cyclic amides are called lactams; they are necessarily secondary or tertiary amides.
Applications
Amides are prevalent throughout the natural and engineered world. Most biological macromolecules consist of peptides linked together through amide bonds; some man-made polymers adopt the same strategy.Properties
Bonding
 The lone pair of
The lone pair of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s on the nitrogen atom is delocalized into the carbonyl group, thus forming a partial double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
between nitrogen and carbon. In fact the O, C and N atoms have molecular orbitals occupied by delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
s, forming a conjugated system. Consequently, the three bonds of the nitrogen in amides is not pyramidal (as in the amines) but planar. This planar restriction prevents rotations about the N linkage and thus has important consequences for the mechanical properties of bulk material of such molecules, and also for the configurational properties of macromolecules built by such bonds. The inability to rotate distinguishes amide groups from ester groups which allow rotation and thus create more flexible bulk material.
The structure of an amide can be described also as a resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system ...
between two alternative structures:
:Basicity
Compared toamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s, amides are very weak bases. While the conjugate acid of an amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
has a p''K''a of about 9.5, the conjugate acid of an amide has a p''K''a around −0.5. Therefore, amides don't have as clearly noticeable acid–base properties in water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
. This relative lack of basicity is explained by the withdrawing of electrons from the amine by the carbonyl. On the other hand, amides are much stronger bases than carboxylic acids, esters, aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s, and ketones (their conjugate acids' p''K''as are between −6 and −10).
The proton of a primary or secondary amide does not dissociate readily under normal conditions; its p''K''a is usually well above 15. Conversely, under extremely acidic conditions, the carbonyl oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
can become protonated with a p''K''a of roughly −1. It is not only because of the positive charge on the nitrogen, but also because of the negative charge on the oxygen gained through resonance.
Hydrogen bonding and solubility
Because of the greater electronegativity of oxygen, the carbonyl (C=O) is a stronger dipole than the N–C dipole. The presence of a C=O dipole and, to a lesser extent a N–C dipole, allows amides to act as H-bond acceptors. In primary and secondary amides, the presence of N–H dipoles allows amides to function as H-bond donors as well. Thus amides can participate inhydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
with water and other protic solvents; the oxygen atom can accept hydrogen bonds from water and the N–H hydrogen atoms can donate H-bonds. As a result of interactions such as these, the water solubility of amides is greater than that of corresponding hydrocarbons. These hydrogen bonds are also have an important role in the secondary structure of proteins.
The solubilities of amides and esters are roughly comparable. Typically amides are less soluble than comparable amines and carboxylic acids since these compounds can both donate and accept hydrogen bonds. Tertiary amides, with the important exception of ''N'',''N''-dimethylformamide, exhibit low solubility in water.
Characterization
The presence of the amide group –C(=O)N– is generally easily established, at least in small molecules. It can be distinguished from nitro and cyano groups in IR spectra. Amides exhibit a moderately intense ''ν''CO band near 1650 cm−1. By 1HNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
, CONHR signals occur at low fields. In X-ray crystallography, the C(=O)N center together with the three immediately adjacent atoms characteristically define a plane.
Reactions
: Amides undergo many chemical reactions, although they are less reactive than esters. Amides hydrolyse in hot alkali as well as in strong acidic conditions. Acidic conditions yield the carboxylic acid and the ammonium ion while basic hydrolysis yield the carboxylate ion and ammonia. The protonation of the initially generated amine under acidic conditions and the deprotonation of the initially generated carboxylic acid under basic conditions render these processes non-catalytic and irreversible. Amides are also versatile precursors to many other functional groups. Electrophiles react with the
Amides undergo many chemical reactions, although they are less reactive than esters. Amides hydrolyse in hot alkali as well as in strong acidic conditions. Acidic conditions yield the carboxylic acid and the ammonium ion while basic hydrolysis yield the carboxylate ion and ammonia. The protonation of the initially generated amine under acidic conditions and the deprotonation of the initially generated carboxylic acid under basic conditions render these processes non-catalytic and irreversible. Amides are also versatile precursors to many other functional groups. Electrophiles react with the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
oxygen. This step often precedes hydrolysis, which is catalyzed by both Brønsted acids and Lewis acids. Enzymes, e.g. peptidase
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the for ...
s and artificial catalysts, are known to accelerate the hydrolysis reactions.
Synthesis
Many methods exist in amide synthesis. Amides can be prepared by coupling carboxylic acid with anamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
. The direct reaction generally requires high temperatures to drive off the water:
:electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
; such as esters, acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s ( Schotten-Baumann reaction), or anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
s ( Lumière–Barbier method).
Conventional methods in peptide synthesis
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl ...
use coupling agents such as HATU, HOBt, or PyBOP.
A variety of reagents, e.g. Tris(2,2,2-trifluoroethyl) borate have been developed for specialized applications.
Other methods
Dehydrogenative acylation of amines is catalyzed by organoruthenium compounds: :aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
followed by formation of a hemiaminal, which undergoes a second dehydrogenation to the amide. Elimination of water in the hemiaminal to the imine is not observed.
Transamidation Transamidation is a chemical reaction in which an amide reacts with an amine to generate a new amide:
:RC(O)NR'2 + HNR"2 → RC(O)NR"2 + HNR'2
The reaction is typically very slow, but it can be accelerated with Lewis acid and organometallic ...
is typically very slow, but it is accelerated with Lewis acid and organometallic catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s:
:See also
* Amidogen *Amino radical
In chemistry, the amino radical, , also known as the aminyl radical or azanyl radical, is the neutral form of the amide ion (). Aminyl radicals are highly reactive and consequently short-lived, like most radicals; however, they form an importa ...
* Amidicity
* Metal amides
References
External links
IUPAC Compendium of Chemical Terminology
{{Authority control Functional groups