Allotropes of sulfur on:
[Wikipedia]
[Google]
[Amazon]
 The element
The element
 The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
 This allotrope was first prepared by M. R. Engel in 1891 by treating
This allotrope was first prepared by M. R. Engel in 1891 by treating
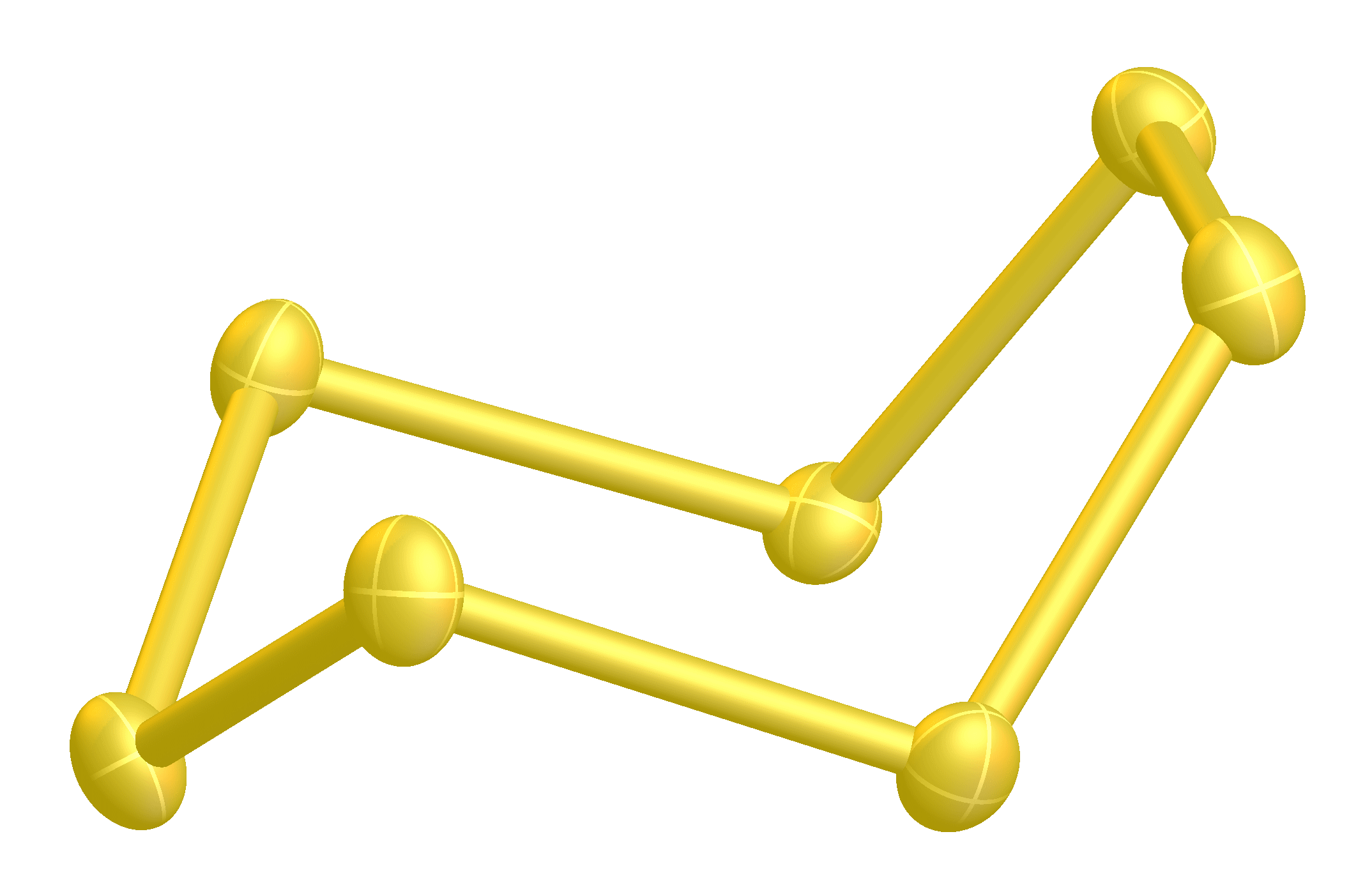 It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known. Greenwood, 657 Two forms (γ-, δ-)have been characterized. The cyclo-S7 ring has an unusual range of bond lengths of 199.3–218.1 pm. It is said to be the least stable of all of the sulfur allotropes. Steudel, 6
It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known. Greenwood, 657 Two forms (γ-, δ-)have been characterized. The cyclo-S7 ring has an unusual range of bond lengths of 199.3–218.1 pm. It is said to be the least stable of all of the sulfur allotropes. Steudel, 6
 These allotropes have been synthesised by various methods for example, treating titanocene pentasulfide and a dichlorosulfane of suitable sulfur chain length, S''n''−5Cl2:
:( η5-C5H5)2TiS5 + S''n''−5Cl2 → cyclo-S''n''+( η5-C5H5)2TiCl2
or alternatively treating a dichlorosulfane, S''n''−''m''Cl2 and a
These allotropes have been synthesised by various methods for example, treating titanocene pentasulfide and a dichlorosulfane of suitable sulfur chain length, S''n''−5Cl2:
:( η5-C5H5)2TiS5 + S''n''−5Cl2 → cyclo-S''n''+( η5-C5H5)2TiCl2
or alternatively treating a dichlorosulfane, S''n''−''m''Cl2 and a
 The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
 The element
The element sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
exists as many allotropes. In number of allotropes, sulfur is second only to carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
. Greenwood, 652 In addition to the allotropes, each allotrope often exists in polymorphs (different crystal structures of the same covalently bonded S molecules) delineated by Greek prefixes (α, β, etc.).
Furthermore, because elemental sulfur has been an item of commerce for centuries, its various forms are given traditional names. Early workers identified some forms that have later proved to be single or mixtures of allotropes. Some forms have been named for their appearance, e.g. "mother of pearl sulfur", or alternatively named for a chemist who was pre-eminent in identifying them, e.g. "Muthmann's sulfur I" or "Engel's sulfur". Steudel, 17
The most commonly encountered form of sulfur is the orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with ...
polymorph of , which adopts a puckered ring – or "crown" – structure. Two other polymorphs are known, also with nearly identical molecular structures. Greenwood, 654 In addition to S8, sulfur rings of 6, 7, 9–15, 18, and 20 atoms are known. Greenwood, 655 At least five allotropes are uniquely formed at high pressures, two of which are metallic.
The number of sulfur allotropes reflects the relatively strong S−S bond of 265 kJ/mol. Furthermore, unlike most elements, the allotropes of sulfur can be manipulated in solutions of organic solvents and are amenable to analysis by HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to p ...
.
Phase diagram
 The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
High pressure solid allotropes
In a high-pressure study at ambient temperatures, four new solid forms, termed II, III, IV, V have been characterized, where α-sulfur is form I. Solid forms II and III are polymeric, while IV and V are metallic (and are superconductive below 10 K and 17 K, respectively). Laser irradiation of solid samples produces three sulfur forms below 200–300 kbar (20–30 GPa).Solid ''cyclo'' allotrope preparation
Two methods exist for the preparation of the ''cyclo''-sulfur allotropes. One of the methods, which is most famous for preparing hexasulfur, is to treat hydrogen polysulfides with polysulfur dichloride: : H2S''x'' + S''y''Cl2 → ''cyclo''-S''x+y'' + 2 HCl A second strategy uses titanocene pentasulfide as a source of the S52− unit. This complex is easily made from polysulfide solutions: : H4sub>2 5+ ( η5-C5H5)2TiCl2 → (C5H5)2TiS5 + 2 NH4Cl Titanocene pentasulfide reacts with polysulfur chloride: : ( η5-C5H5)2TiS5 + S''y''Cl2 → ''cyclo''-S''y''+5 + ( η5-C5H5)2TiCl2Solid cyclo-sulfur allotropes
''Cyclo''-hexasulfur, ''cyclo''-S6
 This allotrope was first prepared by M. R. Engel in 1891 by treating
This allotrope was first prepared by M. R. Engel in 1891 by treating thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e ...
with HCl. Cyclo-S6 is orange-red and forms a rhombohedral crystal. Greenwood, 656 It is called ρ-sulfur, ε-sulfur, Engel's sulfur and Aten's sulfur. Another method of preparation involves the reaction of a polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. Compounds containing 2 – 8 concatenated ...
with sulfur monochloride:
:H2S4 + S2Cl2 → cyclo-S6 + 2 HCl (dilute solution in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
)
The sulfur ring in cyclo-S6 has a "chair" conformation, reminiscent of the chair form of cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohe ...
. All of the sulfur atoms are equivalent.
''Cyclo''-heptasulfur, ''cyclo''-S7
 It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known. Greenwood, 657 Two forms (γ-, δ-)have been characterized. The cyclo-S7 ring has an unusual range of bond lengths of 199.3–218.1 pm. It is said to be the least stable of all of the sulfur allotropes. Steudel, 6
It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known. Greenwood, 657 Two forms (γ-, δ-)have been characterized. The cyclo-S7 ring has an unusual range of bond lengths of 199.3–218.1 pm. It is said to be the least stable of all of the sulfur allotropes. Steudel, 6
''Cyclo''-octasulfur, ''cyclo''-S8
α-Sulfur
α-Sulfur is the form most commonly found in nature. When pure it has a greenish-yellow colour (traces of cyclo-S7 in commercially available samples make it appear yellower). It is practically insoluble in water and is a good electrical insulator with poor thermal conductivity. It is quite soluble incarbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
: 35.5 g/100 g solvent at 25 °C. It has an orthorhombic crystal structure. α-Sulfur is the predominant form found in "flowers of sulfur", "roll sulfur" and "milk of sulfur". It contains S8 puckered rings, alternatively called a crown shape. The S–S bond lengths are all 203.7 pm and the S-S-S angles are 107.8° with a dihedral angle of 98°. At 95.3 °C, α-sulfur converts to β-sulfur.
β-Sulfur
β-Sulfur is a yellow solid with a monoclinic crystal form and is less dense than α-sulfur. Like the α- form it contains puckered S8 rings and only differs from it in the way the rings are packed in the crystal. It is unusual because it is only stable above 95.3 °C; below this temperature it converts to α-sulfur. β-Sulfur can be prepared by crystallising at 100 °C and cooling rapidly to slow down formation of α-sulfur. It has a melting point variously quoted as 119.6 °C and 119.8 °C but as it decomposes to other forms at around this temperature the observed melting point can vary. The 119 °C melting point has been termed the "ideal melting point" and the typical lower value (114.5 °C) when decomposition occurs, the "natural melting point".γ-Sulfur
γ-Sulfur was first prepared by F.W. Muthmann in 1890. It is sometimes called "nacreous sulfur" or "mother of pearl sulfur" because of its appearance. It crystallises in pale yellow monoclinic needles. It contains puckered S8 rings like α-sulfur and β-sulfur and only differs from them in the way that these rings are packed. It is the densest form of the three. It can be prepared by slowly cooling molten sulfur that has been heated above 150 °C or by chilling solutions of sulfur incarbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
, ethyl alcohol or hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s. It is found in nature as the mineral rosickyite. Steudel, 7 It has been tested in carbon fiber-stabilized form as a cathode in lithium-sulfur (Li-S) batteries and was observed to stop the formation of polysulfides that compromise battery life.
''Cyclo''-Sn (n = 9–15, 18, 20)
 These allotropes have been synthesised by various methods for example, treating titanocene pentasulfide and a dichlorosulfane of suitable sulfur chain length, S''n''−5Cl2:
:( η5-C5H5)2TiS5 + S''n''−5Cl2 → cyclo-S''n''+( η5-C5H5)2TiCl2
or alternatively treating a dichlorosulfane, S''n''−''m''Cl2 and a
These allotropes have been synthesised by various methods for example, treating titanocene pentasulfide and a dichlorosulfane of suitable sulfur chain length, S''n''−5Cl2:
:( η5-C5H5)2TiS5 + S''n''−5Cl2 → cyclo-S''n''+( η5-C5H5)2TiCl2
or alternatively treating a dichlorosulfane, S''n''−''m''Cl2 and a polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. Compounds containing 2 – 8 concatenated ...
, H2S''m'':
:S''n''−''m''Cl2 + H2S''m'' → cyclo-S''n''+2 HCl
S12, S18, and S20 can also be prepared from S8. With the exception of cyclo-S12, the rings contain S–S bond lengths and S-S-S bond angle that differ one from another.
Cyclo-S12 is the most stable cyclo-allotrope. Its structure can be visualised as having sulfur atoms in three parallel planes, 3 in the top, 6 in the middle and three in the bottom. Greenwood, 658
Two forms (α-, β-) of cyclo-S9 are known, one of which has been characterized.
Two forms of cyclo-S18 are known where the conformation of the ring is different. To differentiate these structures, rather than using the normal crystallographic convention of α-, β-, etc., which in other cyclo-S''n'' compounds refer to different packings of essentially the same conformer, these two conformers have been termed endo- and exo-.
''Cyclo''-S6.''cyclo''-S10 adduct
This adduct is produced from a solution of cyclo-S6 and cyclo-S10 in CS2. It has a density midway between cyclo-S6 and cyclo-S10. The crystal consists of alternate layers of cyclo-S6 and cyclo-S10. This material is a rare example of an allotrope that contains molecules of different sizes. Steudel, 9Catena sulfur forms
''Catena sulfur forms'' refers to mixtures of sulfur allotropes that are high in catena (polymer chain) sulfur. The naming of the different forms is very confusing and care has to be taken to determine what is being described because some names are used interchangeably.Amorphous sulfur
Amorphous sulfur is the quenched product from molten sulfur hotter than the λ-transition at 160 °C, where polymerization yields catena sulfur molecules. (Above this temperature, the properties of the liquid melt change remarkably. For example, the viscosity increases more than 10000-fold as the temperature increases through the transition). As it anneals, solid amorphous sulfur changes from its initial glassy form, to a plastic form, hence its other names of ''plastic'', and ''glassy'' or ''vitreous'' sulfur. The plastic form is also called χ-sulfur. Amorphous sulfur contains a complex mixture of catena-sulfur forms mixed with cyclo-forms.Insoluble sulfur
Insoluble sulfur is obtained by washing quenched liquid sulfur with CS2. Steudel, 3 It is sometimes called polymeric sulfur, μ-S or ω-S.Fibrous (φ-) sulfur
Fibrous (φ-) sulfur is a mixture of the allotropic ψ- form and γ-cycloS8. Steudel, 43ω-Sulfur
ω-Sulfur is a commercially available product prepared from amorphous sulfur that has not been stretched prior to extraction of soluble forms with CS2. It sometimes called "white sulfur of Das" or supersublimated sulfur. It is a mixture of ψ-sulfur and lamina sulfur. The composition depends on the exact method of production and the sample's history. One well known commercial form is "Crystex". ω-sulfur is used in the vulcanization of rubber. Steudel, 15λ-Sulfur
λ-Sulfur is molten sulfur just above the melting temperature. It is a mixture containing mostly ''cyclo''-S8. Cooling λ-sulfur slowly gives predominantly β-sulfur. Steudel, 26μ-Sulfur
μ-Sulfur is the name applied to solid insoluble sulfur and the melt prior to quenching.π-Sulfur
π-Sulfur is a dark-coloured liquid formed when λ-sulfur is left to stay molten. It contains mixture of Sn rings.Biradical catena (S∞) chains
This term is applied to biradical catena- chains in sulfur melts or the chains in the solid. Greenwood, 662Solid catena allotropes
 The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
ψ-Sulfur
This form, also called fibrous sulfur or ω1-sulfur, has been well characterized. It has a density of 2.01 g·cm−3 (α-sulfur 2.069 g·cm−3) and decomposes around its melting point of 104 °C. It consists of parallel helical sulfur chains. These chains have both left and right-handed "twists" and a radius of 95 pm. The S–S bond length is 206.6 pm, the S-S-S bond angle is 106° and the dihedral angle is 85.3°, (comparable figures for α-sulfur are 203.7 pm, 107.8° and 98.3°). Greenwood, 660Lamina sulfur
Lamina sulfur has not been well characterized but is believed to consist of criss-crossed helices. It is also called χ-sulfur or ω2-sulfur.High-temperature gaseous allotropes
Disulfur, S2
Disulfur, S2, is the predominant species in sulfur vapour above 720 °C (a temperature above that shown in the phase diagram); at low pressure (1 mmHg) at 530 °C, it comprises 99% of the vapor. It is a triplet diradical (like dioxygen and sulfur monoxide), with an S−S bond length of 188.7 pm. The blue colour of burning sulfur is due to the emission of light by the S2 molecule produced in the flame. The S2 molecule has been trapped in the compound 2I4EF6]2 (E = Arsenic, As, Sb) for crystallographic measurements, produced by treating elementalsulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
with excess iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
in liquid sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
. The 2I4sup>2+ cation has an "open-book" structure, in which each 2sup>+ ion donates the unpaired electron in the π* molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of find ...
to a vacant orbital of the S2 molecule.
Trisulfur, S3
S3 is found in sulfur vapour, comprising 10% of vapour species at 440 °C and 10 mmHg. It is cherry red in colour, with a bent structure, similar toozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the l ...
, O3.
Tetrasulfur, S4
S4 has been detected in the vapour phase, but it has not been well characterized. Diverse structures (e.g. chains, branched chains and rings) have been proposed. Theoretical calculations suggest a cyclic structure.Pentasulfur, S5
Pentasulfur has been detected in sulfur vapours but has not been isolated in pure form.List of allotropes and forms
Allotropes are in Bold.References
Bibliography
* *External links
* {{DEFAULTSORT:Sulfur allotropes Amorphous solids