Adhesion on:
[Wikipedia]
[Google]
[Amazon]
 Adhesion is the tendency of dissimilar
Adhesion is the tendency of dissimilar
 Surface energy is conventionally defined as the
Surface energy is conventionally defined as the

 In surface science, the term ''adhesion'' almost always refers to dispersive adhesion. In a typical solid-liquid-gas system (such as a drop of liquid on a solid surrounded by air) the
In surface science, the term ''adhesion'' almost always refers to dispersive adhesion. In a typical solid-liquid-gas system (such as a drop of liquid on a solid surrounded by air) the 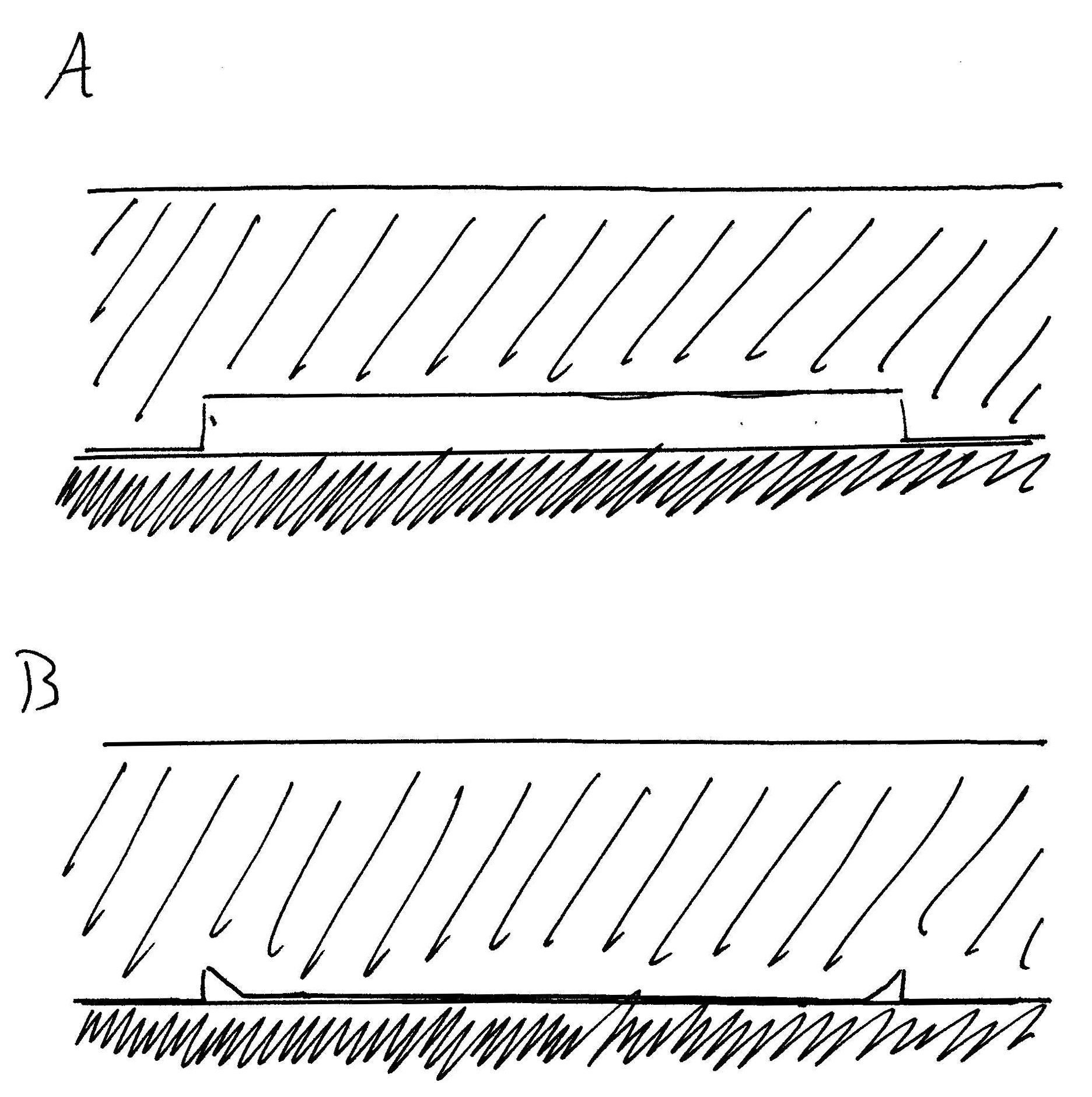 The effect is also apparent in experiments where a
The effect is also apparent in experiments where a
 Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un crosslinked polymers ( thermoplastics), on the other hand are freer to wander into the adjacent phase by extending tails and loops across the interface.
Another circumstance under which diffusive bonding occurs is “scission”.
Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un crosslinked polymers ( thermoplastics), on the other hand are freer to wander into the adjacent phase by extending tails and loops across the interface.
Another circumstance under which diffusive bonding occurs is “scission”.
 Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the stress concentration at the point of failure mentioned earlier. Since the stress is now spread out over some area, the stress at any given point has less of a chance of overwhelming the total adhesive force between the surfaces. If failure does occur at an interface containing a
Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the stress concentration at the point of failure mentioned earlier. Since the stress is now spread out over some area, the stress at any given point has less of a chance of overwhelming the total adhesive force between the surfaces. If failure does occur at an interface containing a
 Adhesion is the tendency of dissimilar
Adhesion is the tendency of dissimilar particle
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from ...
s or surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is ...
s to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
The forces that cause adhesion and cohesion can be divided into several types. The intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. ...
s responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion
Dispersive adhesion, also called adsorptive adhesion, is a mechanism for adhesion which attributes attractive forces between two materials to intermolecular interactions between molecules of each material. This mechanism is widely viewed as th ...
, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects.
Surface energy
 Surface energy is conventionally defined as the
Surface energy is conventionally defined as the work
Work may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
** Manual labour, physical work done by humans
** House work, housework, or homemaking
** Working animal, an animal t ...
that is required to build an area of a particular surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is ...
. Another way to view the surface energy is to relate it to the work required to cleave a bulk sample, creating two surfaces. If the new surfaces are identical, the surface energy γ of each surface is equal to half the work of cleavage, W: γ = (1/2)W11.
If the surfaces are unequal, the Young-Dupré equation applies:
W12 = γ1 + γ2 – γ12, where γ1 and γ2 are the surface energies of the two new surfaces, and γ12 is the interfacial energy.
This methodology can also be used to discuss cleavage that happens in another medium: γ12 = (1/2)W121 = (1/2)W212. These two energy quantities refer to the energy that is needed to cleave one species into two pieces while it is contained in a medium of the other species. Likewise for a three species system: γ13 + γ23 – γ12 = W12 + W33 – W13 – W23 = W132, where W132 is the energy of cleaving species 1 from species 2 in a medium of species 3.
A basic understanding of the terminology of cleavage energy, surface energy, and surface tension is very helpful for understanding the physical state
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, ...
and the events that happen at a given surface, but as discussed below, the theory of these variables also yields some interesting effects that concern the practicality of adhesive surfaces in relation to their surroundings.J. N. Israelachvili, ''Intermolecular and Surface Forces'' (Academic Press, New York, 1985). chap. 15.
Mechanisms
There is no single theory covering adhesion, and particular mechanisms are specific to particular material scenarios. Five mechanisms of adhesion have been proposed to explain why one material sticks to another:Mechanical
Adhesive materials fill the voids or pores of the surfaces and hold surfaces together byinterlocking
In railway signalling, an interlocking is an arrangement of signal apparatus that prevents conflicting movements through an arrangement of tracks such as junctions or crossings. The signalling appliances and tracks are sometimes collectively re ...
. Other interlocking phenomena are observed on different length scales. Sewing is an example of two materials forming a large scale mechanical bond, velcro
Velcro, officially known as Velcro IP Holdings LLC and trading as Velcro Companies, is a British privately held company, founded by Swiss electrical engineer George de Mestral in the 1950s. It is the original manufacturer of hook-and-loop fast ...
forms one on a medium scale, and some textile adhesives (glue) form one at a small scale.
Chemical
Two materials may form acompound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
at the joint. The strongest joints are where atoms of the two materials share or swap electrons (known respectively as covalent bonding
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
or ionic bonding
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
). A weaker bond is formed if a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom in one molecule is attracted to an atom of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, or fluorine in another molecule, a phenomenon called hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
.
Chemical adhesion occurs when the surface atoms of two separate surfaces form ionic, covalent, or hydrogen bonds. The engineering principle behind chemical adhesion in this sense is fairly straightforward: if surface molecules can bond, then the surfaces will be bonded together by a network of these bonds. It bears mentioning that these attractive ionic and covalent forces are effective over only very small distances – less than a nanometer. This means in general not only that surfaces with the potential for chemical bonding need to be brought very close together, but also that these bonds are fairly brittle, since the surfaces then need to be kept close together.
Dispersive
In dispersive adhesion, also known as physisorption, two materials are held together by van der Waals forces: the attraction between two molecules, each of which has a region of slight positive and negative charge. In the simple case, such molecules are therefore polar with respect to average charge density, although in larger or more complex molecules, there may be multiple "poles" or regions of greater positive or negative charge. These positive and negative poles may be a permanent property of a molecule (Keesom force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s) or a transient effect which can occur in any molecule, as the random movement of electrons within the molecules may result in a temporary concentration of electrons in one region ( London forces).

 In surface science, the term ''adhesion'' almost always refers to dispersive adhesion. In a typical solid-liquid-gas system (such as a drop of liquid on a solid surrounded by air) the
In surface science, the term ''adhesion'' almost always refers to dispersive adhesion. In a typical solid-liquid-gas system (such as a drop of liquid on a solid surrounded by air) the contact angle
The contact angle is the angle, conventionally measured through the liquid, where a liquid–vapor interface meets a solid surface. It quantifies the wettability of a solid surface by a liquid via the Young equation. A given system of solid, liq ...
is used to evaluate adhesiveness indirectly, while a Centrifugal Adhesion Balance allows for direct quantitative adhesion measurements. Generally, cases where the contact angle is low are considered of higher adhesion per unit area. This approach assumes that the lower contact angle corresponds to a higher surface energy. Theoretically, the more exact relation between contact angle and work of adhesion is more involved and is given by the Young-Dupre equation
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with th ...
. The contact angle of the three-phase system is a function not only of dispersive adhesion (interaction between the molecules in the liquid and the molecules in the solid) but also cohesion (interaction between the liquid molecules themselves). Strong adhesion and weak cohesion results in a high degree of wetting, a lyophilic condition with low measured contact angles. Conversely, weak adhesion and strong cohesion results in lyophobic conditions with high measured contact angles and poor wetting.
London dispersion forces are particularly useful for the function of adhesive devices, because they don't require either surface to have any permanent polarity. They were described in the 1930s by Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces ( London dispersion forces) are today ...
, and have been observed by many researchers. Dispersive forces are a consequence of statistical quantum mechanics. London theorized that attractive forces between molecules that cannot be explained by ionic or covalent interaction can be caused by polar moments within molecules. Multipoles could account for attraction between molecules having permanent multipole moments that participate in electrostatic interaction
Electrostatics is a branch of physics that studies electric charges at rest (static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after triboelectric effect, rubbing. ...
. However, experimental data showed that many of the compounds observed to experience van der Waals forces had no multipoles at all. London suggested that momentary dipoles are induced purely by virtue of molecules being in proximity to one another. By solving the quantum mechanical system of two electrons as harmonic oscillators at some finite distance from one another, being displaced about their respective rest positions and interacting with each other's fields, London showed that the energy of this system is given by:
:
While the first term is simply the zero-point energy, the negative second term describes an attractive force between neighboring oscillators. The same argument can also be extended to a large number of coupled oscillators, and thus skirts issues that would negate the large scale attractive effects of permanent dipoles cancelling through symmetry, in particular.
The additive nature of the dispersion effect has another useful consequence. Consider a single such dispersive dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system ...
, referred to as the origin dipole. Since any origin dipole is inherently oriented so as to be attracted to the adjacent dipoles it induces, while the other, more distant dipoles are not correlated with the original dipole by any phase relation (thus on average contributing nothing), there is a net attractive force in a bulk of such particles. When considering identical particles, this is called cohesive force.F. London, "The General Theory of Molecular Forces" (1936).
When discussing adhesion, this theory needs to be converted into terms relating to surfaces. If there is a net attractive energy of cohesion in a bulk of similar molecules, then cleaving this bulk to produce two surfaces will yield surfaces with a dispersive surface energy, since the form of the energy remain the same. This theory provides a basis for the existence of van der Waals forces at the surface, which exist between any molecules having electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
. These forces are easily observed through the spontaneous jumping of smooth surfaces into contact. Smooth surfaces of mica, gold, various polymers and solid gelatin solutions do not stay apart when their separating becomes small enough – on the order of 1–10 nm. The equation describing these attractions was predicted in the 1930s by De Boer and Hamaker:
:
where P is the force (negative for attraction), z is the separation distance, and A is a material-specific constant called the Hamaker constant
The Hamaker constant ''A'' can be defined for a van der Waals (vdW) body–body interaction:
:A=\pi^2C\rho_1\rho_2,
where \rho_1 and \rho_2 are the number densities of the two interacting kinds of particles, and ''C'' is the London coefficient in ...
.
 The effect is also apparent in experiments where a
The effect is also apparent in experiments where a polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as its ...
(PDMS) stamp is made with small periodic post structures. The surface with the posts is placed face down on a smooth surface, such that the surface area in between each post is elevated above the smooth surface, like a roof supported by columns. Because of these attractive dispersive forces between the PDMS and the smooth substrate, the elevated surface – or “roof” – collapses down onto the substrate without any external force aside from the van der Waals attraction. Simple smooth polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
surfaces – without any microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymers ...
s – are commonly used for these dispersive adhesive properties. Decals and stickers that adhere to glass without using any chemical adhesives are fairly common as toys and decorations and useful as removable labels because they do not rapidly lose their adhesive properties, as do sticky tapes that use adhesive chemical compounds.
It is important to note that these forces also act over very small distances – 99% of the work necessary to break van der Waals bonds is done once surfaces are pulled more than a nanometer apart. As a result of this limited motion in both the van der Waals and ionic/covalent bonding situations, practical effectiveness of adhesion due to either or both of these interactions leaves much to be desired. Once a crack is initiated, it propagates easily along the interface because of the brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. Br ...
nature of the interfacial bonds.
As an additional consequence, increasing surface area often does little to enhance the strength of the adhesion in this situation. This follows from the aforementioned crack failure – the stress at the interface is not uniformly distributed, but rather concentrated at the area of failure.
Electrostatic
Some conducting materials may pass electrons to form a difference inelectrical charge
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described ...
at the joint. This results in a structure similar to a capacitor
A capacitor is a device that stores electrical energy in an electric field by virtue of accumulating electric charges on two close surfaces insulated from each other. It is a passive electronic component with two terminals.
The effect of ...
and creates an attractive electrostatic force between the materials.
Diffusive
Some materials may merge at the joint bydiffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
. This may occur when the molecules of both materials are mobile and soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in each other. This would be particularly effective with polymer chains where one end of the molecule diffuses into the other material. It is also the mechanism involved in sintering. When metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
or ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain ...
powders are pressed together and heated, atoms diffuse from one particle to the next. This joins the particles into one.
 Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un crosslinked polymers ( thermoplastics), on the other hand are freer to wander into the adjacent phase by extending tails and loops across the interface.
Another circumstance under which diffusive bonding occurs is “scission”.
Diffusive forces are somewhat like mechanical tethering at the molecular level. Diffusive bonding occurs when species from one surface penetrate into an adjacent surface while still being bound to the phase of their surface of origin. One instructive example is that of polymer-on-polymer surfaces. Diffusive bonding in polymer-on-polymer surfaces is the result of sections of polymer chains from one surface interdigitating with those of an adjacent surface. The freedom of movement of the polymers has a strong effect on their ability to interdigitate, and hence, on diffusive bonding. For example, cross-linked polymers are less capable of diffusion and interdigitation because they are bonded together at many points of contact, and are not free to twist into the adjacent surface. Un crosslinked polymers ( thermoplastics), on the other hand are freer to wander into the adjacent phase by extending tails and loops across the interface.
Another circumstance under which diffusive bonding occurs is “scission”. Chain scission
Chain scission is a term used in polymer chemistry describing the Fracture in polymers, degradation of a polymer main chain. It is often caused by Thermal degradation of polymers, thermal stress (heat) or ionizing radiation (e.g. light, UV radiatio ...
is the cutting up of polymer chains, resulting in a higher concentration of distal tails. The heightened concentration of these chain ends gives rise to a heightened concentration of polymer tails extending across the interface. Scission is easily achieved by ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
irradiation in the presence of oxygen gas, which suggests that adhesive devices employing diffusive bonding actually benefit from prolonged exposure to heat/light and air. The longer such a device is exposed to these conditions, the more tails are scissed and branch out across the interface.
Once across the interface, the tails and loops form whatever bonds are favorable. In the case of polymer-on-polymer surfaces, this means more van der Waals forces. While these may be brittle, they are quite strong when a large network of these bonds is formed. The outermost layer of each surface plays a crucial role in the adhesive properties of such interfaces, as even a tiny amount of interdigitation – as little as one or two tails of 1.25 angstrom length – can increase the van der Waals bonds by an order of magnitude.
Strength
The strength of the adhesion between two materials depends on which of the above mechanisms occur between the two materials, and the surface area over which the two materials contact. Materials that wet against each other tend to have a larger contact area than those that do not. Wetting depends on the surface energy of the materials. Low surface energy materials such aspolyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including b ...
, polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.
Polypropylene
belongs to the group of polyolefins a ...
, polytetrafluoroethylene and polyoxymethylene
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. As with many other synthetic pol ...
are difficult to bond without special surface preparation.
Another factor determining the strength of an adhesive contact is its shape. Adhesive contacts of complex shape begin to detach at the "edges" of the contact area. The process of destruction of adhesive contacts can be seen in the film.
Other effects
In concert with the primary surface forces described above, there are several circumstantial effects in play. While the forces themselves each contribute to the magnitude of the adhesion between the surfaces, the following play a crucial role in the overall strength and reliability of an adhesive device.Stringing
 Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the stress concentration at the point of failure mentioned earlier. Since the stress is now spread out over some area, the stress at any given point has less of a chance of overwhelming the total adhesive force between the surfaces. If failure does occur at an interface containing a
Stringing is perhaps the most crucial of these effects, and is often seen on adhesive tapes. Stringing occurs when a separation of two surfaces is beginning and molecules at the interface bridge out across the gap, rather than cracking like the interface itself. The most significant consequence of this effect is the restraint of the crack. By providing the otherwise brittle interfacial bonds with some flexibility, the molecules that are stringing across the gap can stop the crack from propagating. Another way to understand this phenomenon is by comparing it to the stress concentration at the point of failure mentioned earlier. Since the stress is now spread out over some area, the stress at any given point has less of a chance of overwhelming the total adhesive force between the surfaces. If failure does occur at an interface containing a viscoelastic
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like water, resist shear flow and strain linearly ...
adhesive agent, and a crack does propagate, it happens by a gradual process called “fingering”, rather than a rapid, brittle fracture.
Stringing can apply to both the diffusive bonding regime and the chemical bonding regime. The strings of molecules bridging across the gap would either be the molecules that had earlier diffused across the interface or the viscoelastic adhesive, provided that there was a significant volume of it at the interface.
Microstructures
The interplay of molecular scale mechanisms and hierarchical surface structures is known to result in high levels of static friction and bonding between pairs of surfaces. Technologically advanced adhesive devices sometimes make use of microstructures on surfaces, such as tightly packed periodic posts. These arebiomimetic
Biomimetics or biomimicry is the emulation of the models, systems, and elements of nature for the purpose of solving complex human problems. The terms "biomimetics" and "biomimicry" are derived from grc, βίος (''bios''), life, and μίμησ ...
technologies inspired by the adhesive abilities of the feet of various arthropod
Arthropods (, (gen. ποδός)) are invertebrate animals with an exoskeleton, a segmented body, and paired jointed appendages. Arthropods form the phylum Arthropoda. They are distinguished by their jointed limbs and cuticle made of chiti ...
s and vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () (chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, with c ...
s (most notably, geckos). By intermixing periodic breaks into smooth, adhesive surfaces, the interface acquires valuable crack-arresting properties. Because crack initiation requires much greater stress than does crack propagation, surfaces like these are much harder to separate, as a new crack has to be restarted every time the next individual microstructure is reached.
Hysteresis
Hysteresis, in this case, refers to the restructuring of the adhesive interface over some period of time, with the result being that the work needed to separate two surfaces is greater than the work that was gained by bringing them together (W > γ1 + γ2). For the most part, this is a phenomenon associated with diffusive bonding. The more time is given for a pair of surfaces exhibiting diffusive bonding to restructure, the more diffusion will occur, the stronger the adhesion will become. The aforementioned reaction of certain polymer-on-polymer surfaces to ultraviolet radiation and oxygen gas is an instance of hysteresis, but it will also happen over time without those factors. In addition to being able to observe hysteresis by determining if W > γ1 + γ2 is true, one can also find evidence of it by performing “stop-start” measurements. In these experiments, two surfaces slide against one another continuously and occasionally stopped for some measured amount of time. Results from experiments on polymer-on-polymer surfaces show that if the stopping time is short enough, resumption of smooth sliding is easy. If, however, the stopping time exceeds some limit, there is an initial increase of resistance to motion, indicating that the stopping time was sufficient for the surfaces to restructure.Wettability and absorption
Some atmospheric effects on the functionality of adhesive devices can be characterized by following the theory of surface energy and interfacial tension. It is known that γ12 = (1/2)W121 = (1/2)W212. If γ12 is high, then each species finds it favorable to cohere while in contact with a foreign species, rather than dissociate and mix with the other. If this is true, then it follows that when the interfacial tension is high, the force of adhesion is weak, since each species does not find it favorable to bond to the other. The interfacial tension of a liquid and a solid is directly related to the liquid'swettability
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with ...
(relative to the solid), and thus one can extrapolate that cohesion increases in non-wetting liquids and decreases in wetting liquids. One example that verifies this is polydimethyl siloxane rubber, which has a work of self-adhesion of 43.6 mJ/m2 in air, 74 mJ/m2 in water (a nonwetting liquid) and 6 mJ/m2 in methanol (a wetting liquid).
This argument can be extended to the idea that when a surface is in a medium with which binding is favorable, it will be less likely to adhere to another surface, since the medium is taking up the potential sites on the surface that would otherwise be available to adhere to another surface. Naturally this applies very strongly to wetting liquids, but also to gas molecules that could adsorb onto the surface in question, thereby occupying potential adhesion sites. This last point is actually fairly intuitive: Leaving an adhesive exposed to air too long gets it dirty, and its adhesive strength will decrease. This is observed in the experiment: when mica is cleaved in air, its cleavage energy, W121 or Wmica/air/mica, is smaller than the cleavage energy in vacuum, Wmica/vac/mica, by a factor of 13.
Lateral adhesion
Lateral adhesion is the adhesion associated with sliding one object on a substrate such as sliding a drop on a surface. When the two objects are solids, either with or without a liquid between them, the lateral adhesion is described asfriction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction:
*Dry friction is a force that opposes the relative lateral motion of ...
. However, the behavior of lateral adhesion between a drop and a surface is tribologically very different from friction between solids, and the naturally adhesive contact between a flat surface and a liquid drop makes the lateral adhesion in this case, an individual field. Lateral adhesion can be measured using the centrifugal adhesion balance (CAB), which uses a combination of centrifugal and gravitational forces to decouple the normal and lateral forces in the problem.
See also
* Adhesive * Adhesive bonding *Bacterial adhesin Adhesins are cell-surface components or appendages of bacteria that facilitate adhesion or adherence to other cells or to surfaces, usually in the host they are infecting or living in. Adhesins are a type of virulence factor.
Adherence is an essen ...
* Capillary action
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces li ...
* Cell adhesion
* Contact mechanics
Contact mechanics is the study of the deformation of solids that touch each other at one or more points.Johnson, K. L, 1985, Contact mechanics, Cambridge University Press.Popov, Valentin L., 2010, ''Contact Mechanics and Friction. Physical P ...
* Fracture mechanics
* Galling
* Insect adhesion
* Meniscus
* Mucoadhesion Mucoadhesion describes the attractive forces between a biological material and mucus or mucous membrane. Mucous membranes adhere to epithelial surfaces such as the gastrointestinal tract (GI-tract), the vagina, the lung, the eye, etc. They are gener ...
* Pressure-sensitive adhesive
* Rail adhesion
An adhesion railway relies on adhesion traction to move the train. Adhesion traction is the friction between the drive wheels and the steel rail. The term "adhesion railway" is used only when it is necessary to distinguish adhesion railways from ...
* Synthetic setae
* Wetting
* Cohesion number
References
Further reading
* John Comyn, ''Adhesion Science'', Royal Society of Chemistry Paperbacks, 1997 * A.J. Kinloch, ''Adhesion and Adhesives: Science and Technology'', Chapman and Hall, 1987 {{Authority control Materials science Chemical properties Intermolecular forces Articles containing video clips