Adenosyl-fluoride synthase on:
[Wikipedia]
[Google]
[Amazon]
The fluorinase enzyme (, also known as adenosyl-fluoride synthase) 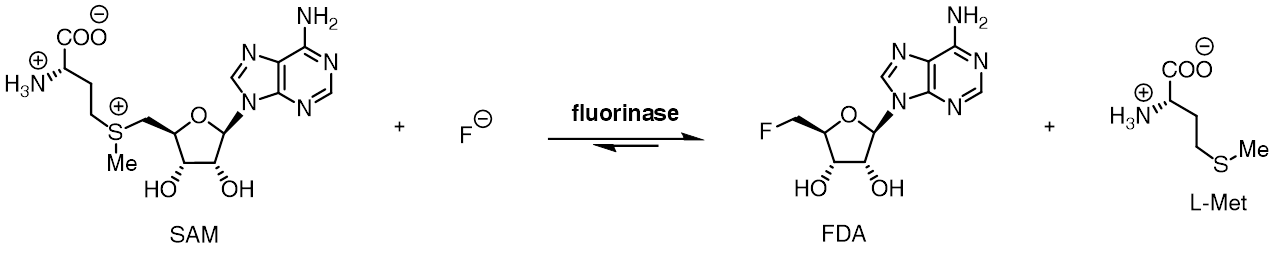 A homologous chlorinase enzyme, which catalyses the same reaction with chloride rather than fluoride ion, has been isolated from ''Salinospora tropica'', from the biosynthetic pathway of salinosporamide A.
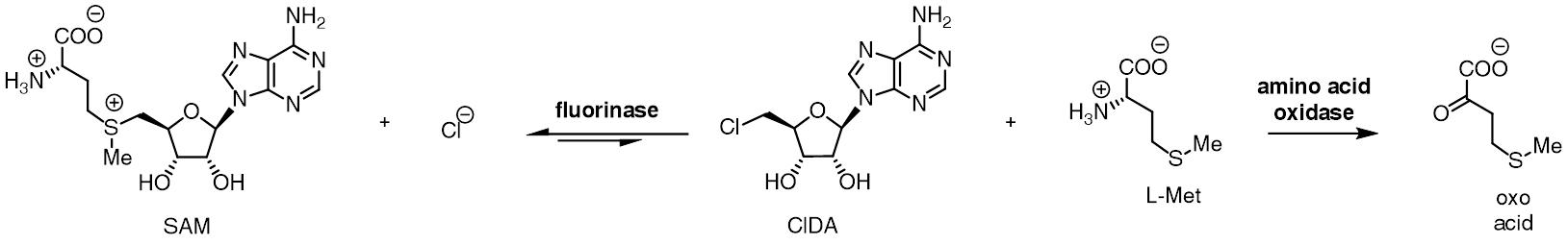
A homologous chlorinase enzyme, which catalyses the same reaction with chloride rather than fluoride ion, has been isolated from ''Salinospora tropica'', from the biosynthetic pathway of salinosporamide A.
 The halide preference, coupled to the position of the two reaction equilibria allows for a nett transhalogenation reaction to be catalysed by the enzyme. Incubation of 5'-chloro nucleosides with the enzyme, along with catalytic L-selenomethionine or L-methionine results in the production of 5-fluoro nucleosides. When sup>18Fluoride is used, this transhalogenation reaction can be used for the synthesis of
The halide preference, coupled to the position of the two reaction equilibria allows for a nett transhalogenation reaction to be catalysed by the enzyme. Incubation of 5'-chloro nucleosides with the enzyme, along with catalytic L-selenomethionine or L-methionine results in the production of 5-fluoro nucleosides. When sup>18Fluoride is used, this transhalogenation reaction can be used for the synthesis of 
catalyzes
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
the reaction between fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
ion and the co-factor '' S'' -adenosyl-L-methionine to generate L-methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical rol ...
and 5'-fluoro-5'-deoxyadenosine, the first committed product of the fluorometabolite biosynthesis pathway. The fluorinase was originally isolated from the soil bacterium ''Streptomyces cattleya
''Streptomyces cattleya'' is a Gram-positive bacterium which makes cephamycin, penicillin and thienamycin. The bacterium expresses a fluorinase enzyme, and the organism has been used to understand the biosynthesis of fluoroacetate and the antibac ...
'', but homologues have since been identified in a number of other bacterial species, including ''Streptomyces'' sp. MA37, '' Nocardia brasiliensis'' and ''Actinoplanes
''Actinoplanes'' is a genus in the family Micromonosporaceae. They have aerial mycelia and spherical, motile spores. ''Actinoplanes'' species produce the pharmaceutically important compounds valienamine (a precursor to the antidiabetic drug acarb ...
'' sp. N902-109. This is the only known enzyme capable of catalysing the formation of a carbon-fluorine bond, the strongest single bond in organic chemistry.
 A homologous chlorinase enzyme, which catalyses the same reaction with chloride rather than fluoride ion, has been isolated from ''Salinospora tropica'', from the biosynthetic pathway of salinosporamide A.
A homologous chlorinase enzyme, which catalyses the same reaction with chloride rather than fluoride ion, has been isolated from ''Salinospora tropica'', from the biosynthetic pathway of salinosporamide A.
Reactivity
The fluorinase catalyses an SN2-type nucleophilic substitution at the C-5' position of SAM, while L-methionine acts as a neutral leaving group. The fluorinase-catalysed reaction is estimated to be between 106 to 1015 times faster than the uncatalysed reaction, a significant rate enhancement. Despite this, the fluorinase is still regarded as a slow enzyme, with aturnover number Turnover number has two different meanings:
In enzymology, turnover number (also termed ''k''cat) is defined as the maximum number of chemical conversions of substrate molecules per second that a single active site will execute for a given enzyme ...
(''k''cat) of 0.06 min−1. The high kinetic barrier to reaction is attributed to the strong solvation of fluoride ion in water, resulting in a high activation energy associated with stripping solvating water molecules from aqueous fluoride ion, converting fluoride into a potent nucleophile within the active site.
The reaction catalysed by the fluorinase is reversible, and upon incubation of 5'-fluoro-5'-deoxyadenosine and L-methionine with the fluorinase, SAM and fluoride ion are produced. Replacing L-methionine with L-selenomethionine results in a 6-fold rate enhancement of the reverse reaction, due to the increased nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
of the selenium centre compared to the sulfur centre.
The fluorinase shows a degree of substrate tolerance for halide ion, and can also use chloride ion in place of fluoride ion. While the equilibrium for reaction between SAM and fluoride ion lies towards products FDA and L-methionine, the equilibrium position is reversed in the case for chloride ion. Incubation of SAM and chloride ion with the fluorinase does not result in generation of 5'-chloro-5'-deoxyadenosine (ClDA), unless an additional enzyme, an L- amino acid oxidase, is added. The amino acid oxidase removes the L-methionine from the reaction, converting it to the corresponding oxo-acid.
 The halide preference, coupled to the position of the two reaction equilibria allows for a nett transhalogenation reaction to be catalysed by the enzyme. Incubation of 5'-chloro nucleosides with the enzyme, along with catalytic L-selenomethionine or L-methionine results in the production of 5-fluoro nucleosides. When sup>18Fluoride is used, this transhalogenation reaction can be used for the synthesis of
The halide preference, coupled to the position of the two reaction equilibria allows for a nett transhalogenation reaction to be catalysed by the enzyme. Incubation of 5'-chloro nucleosides with the enzyme, along with catalytic L-selenomethionine or L-methionine results in the production of 5-fluoro nucleosides. When sup>18Fluoride is used, this transhalogenation reaction can be used for the synthesis of radiotracers
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tr ...
for positron emission tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including bl ...
.

Structural studies
As of late 2007, 9structures
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
have been solved for this class of enzymes, with PDB accession codes , , , , , , , , and .
The names given to the enzyme come not from the structure, but from the function: 5-Fluoro-5-deoxyadenosine is the molecule synthesised. The structure is homologous to the duf-62 enzyme series. The enzyme is a dimer of trimers (2 molecules each with three subunits). The active sites are located between these subunits
(subunit interfaces), each can bind to one SAM molecule at a time.
Fluorometabolite biosynthesis
See also
*Carbon–fluorine bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F s ...
* Organofluorine
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refri ...
References
{{Portal bar, Biology, border=no Enzymes EC 2.5.1