Actinium on:
[Wikipedia]
[Google]
[Amazon]
Actinium is a
 Actinium is found only in traces in
Actinium is found only in traces in ^_Ra + ^_n -> ^_Ra -> beta^-42.2 \ \ce] ^_Ac
The reaction yield is about 2% of the radium weight. 227Ac can further capture neutrons resulting in small amounts of 228Ac. After the synthesis, actinium is separated from radium and from the products of decay and nuclear fusion, such as thorium, polonium, lead and bismuth. The extraction can be performed with thenoyltrifluoroacetone-
''Structure-property relations in nonferrous metals''
Wiley. , pp. 470–471 In all those applications, 227Ac (a beta source) is merely a progenitor which generates alpha-emitting isotopes upon its decay. Beryllium captures alpha particles and emits neutrons owing to its large cross-section for the (α,n) nuclear reaction: :^_Be + ^_He -> ^_C + ^_n + \gamma
The 227AcBe neutron sources can be applied in a neutron probe – a standard device for measuring the quantity of water present in soil, as well as moisture/density for quality control in highway construction. Such probes are also used in well logging applications, in neutron radiography, tomography and other radiochemical investigations.
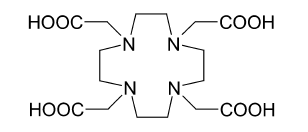 225Ac is applied in medicine to produce in a reusable generator or can be used alone as an agent for
225Ac is applied in medicine to produce in a reusable generator or can be used alone as an agent for
''Synthesis of lanthanide and actinide compounds''
Springer.
at ''
NLM Hazardous Substances Databank – Actinium, Radioactive
Actinium
in {{Good article Chemical elements Chemical elements with face-centered cubic structure Actinides
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Ac and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
89. It was first isolated by Friedrich Oskar Giesel
Friedrich Oskar Giesel (20 May 1852 – 13 November 1927, known as Fritz) was a German organic chemist. During his work in a quinine factory in the late 1890s, he started to work on the at-that-time-new field of radiochemistry and started the ...
in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance André-Louis Debierne
André-Louis Debierne (; 14 July 1874 – 31 August 1949) was a French chemist. He is often considered the discoverer of the element actinium, though H. W. Kirby disputes this and awards credit instead to German chemist Friedrich Oskar Giesel.
D ...
found in 1899 and called actinium. Actinium gave the name to the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
series, a group of 15 similar elements between actinium and lawrencium
Lawrencium is a synthetic chemical element with the symbol Lr (formerly Lw) and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radio ...
in the periodic table. Together with polonium
Polonium is a chemical element with the symbol Po and atomic number 84. Polonium is a chalcogen. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character ...
, radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.
A soft, silvery-white radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and many actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s, actinium assumes oxidation state +3 in nearly all its chemical compounds. Actinium is found only in traces in uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
ores as the isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
227Ac, which decays with a half-life of 21.772 years, predominantly emitting beta and sometimes alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pr ...
s, and 228Ac, which is beta active with a half-life of 6.15 hours. One tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
of natural uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
in ore contains about 0.2 milligrams of actinium-227, and one tonne of thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
contains about 5 nanograms of actinium-228. The close similarity of physical and chemical properties of actinium and lanthanum makes separation of actinium from the ore impractical. Instead, the element is prepared, in milligram amounts, by the neutron irradiation of in a nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
. Owing to its scarcity, high price and radioactivity, actinium has no significant industrial use. Its current applications include a neutron source and an agent for radiation therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radi ...
.
History
André-Louis Debierne
André-Louis Debierne (; 14 July 1874 – 31 August 1949) was a French chemist. He is often considered the discoverer of the element actinium, though H. W. Kirby disputes this and awards credit instead to German chemist Friedrich Oskar Giesel.
D ...
, a French chemist, announced the discovery of a new element in 1899. He separated it from pitchblende
Uraninite, formerly pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2 but because of oxidation typically contains variable proportions of U3O8. Radioactive decay of the uranium causes t ...
residues left by Marie
Marie may refer to:
People Name
* Marie (given name)
* Marie (Japanese given name)
* Marie (murder victim), girl who was killed in Florida after being pushed in front of a moving vehicle in 1973
* Marie (died 1759), an enslaved Cree person in Tr ...
and Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becq ...
after they had extracted radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
. In 1899, Debierne described the substance as similar to titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
and (in 1900) as similar to thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
. Friedrich Oskar Giesel
Friedrich Oskar Giesel (20 May 1852 – 13 November 1927, known as Fritz) was a German organic chemist. During his work in a quinine factory in the late 1890s, he started to work on the at-that-time-new field of radiochemistry and started the ...
found in 1902 a substance similar to lanthanum and called it "emanium" in 1904. After a comparison of the substances' half-lives determined by Debierne, Harriet Brooks in 1904, and Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the fields of radioactivity and radiochemistry. He is referred to as the father of nuclear chemistry and father of nuclear fission. Hahn and Lise Meitner ...
and Otto Sackur in 1905, Debierne's chosen name for the new element was retained because it had seniority, despite the contradicting chemical properties he claimed for the element at different times.
Articles published in the 1970s and later suggest that Debierne's results published in 1904 conflict with those reported in 1899 and 1900. Furthermore, the now-known chemistry of actinium precludes its presence as anything other than a minor constituent of Debierne's 1899 and 1900 results; in fact, the chemical properties he reported make it likely that he had, instead, accidentally identified protactinium
Protactinium (formerly protoactinium) is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds ...
, which would not be discovered for another fourteen years, only to have it disappear due to its hydrolysis and adsorption onto his laboratory equipment. This has led some authors to advocate that Giesel alone should be credited with the discovery. A less confrontational vision of scientific discovery is proposed by Adloff. He suggests that hindsight criticism of the early publications should be mitigated by the then nascent state of radiochemistry: highlighting the prudence of Debierne's claims in the original papers, he notes that nobody can contend that Debierne's substance did not contain actinium. Debierne, who is now considered by the vast majority of historians as the discoverer, lost interest in the element and left the topic. Giesel, on the other hand, can rightfully be credited with the first preparation of radiochemically pure actinium and with the identification of its atomic number 89.
The name actinium originates from the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
''aktis, aktinos'' (ακτίς, ακτίνος), meaning beam or ray. Its symbol Ac is also used in abbreviations of other compounds that have nothing to do with actinium, such as acetyl, acetate and sometimes acetaldehyde.
Properties
Actinium is a soft, silvery-white,''Actinium'', in Encyclopædia Britannica, 15th edition, 1995, p. 70radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
, metallic element. Its estimated shear modulus
In materials science, shear modulus or modulus of rigidity, denoted by ''G'', or sometimes ''S'' or ''μ'', is a measure of the elastic shear stiffness of a material and is defined as the ratio of shear stress to the shear strain:
:G \ \stackre ...
is similar to that of lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
. Owing to its strong radioactivity, actinium glows in the dark with a pale blue light, which originates from the surrounding air ionized by the emitted energetic particles. Actinium has similar chemical properties to lanthanum and other lanthanides, and therefore these elements are difficult to separate when extracting from uranium ores. Solvent extraction and ion chromatography are commonly used for the separation.
The first element of the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s, actinium gave the set its name, much as lanthanum had done for the lanthanides. The actinides are much more diverse than the lanthanides and therefore it was not until 1945 that the most significant change to Dmitri Mendeleev's periodic table since the recognition of the lanthanides, the introduction of the actinides, was generally accepted after Glenn T. Seaborg's research on the transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s (although it had been proposed as early as 1892 by British chemist Henry Bassett).
Actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that impedes further oxidation. As with most lanthanides and actinides, actinium exists in the oxidation state +3, and the Ac3+ ions are colorless in solutions. The oxidation state +3 originates from the nd17s2 electronic configuration of actinium, with three valence electrons that are easily donated to give the stable closed-shell structure of the noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
radon. Although the 5f orbitals are unoccupied in an actinium atom, it can be used as a valence orbital in actinium complexes and hence actinium is generally regarded by chemists working on it as the first 5f element. Ac3+ is the largest of all known tripositive ions and its first coordination sphere contains approximately 10.9 ± 0.5 water molecules.
Chemical compounds
Due to actinium's intense radioactivity, only a limited number of actinium compounds are known. These include: AcF3, AcCl3, AcBr3, AcOF, AcOCl, AcOBr, Ac2S3, Ac2O3, AcPO4 and Ac(NO3)3. Except for AcPO4, they are all similar to the corresponding lanthanum compounds. They all contain actinium in the oxidation state +3. In particular, the lattice constants of the analogous lanthanum and actinium compounds differ by only a few percent. Here ''a'', ''b'' and ''c'' are lattice constants, No is space group number and ''Z'' is the number offormula unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. E ...
s per unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
. Density was not measured directly but calculated from the lattice parameters.
Oxides
Actinium oxide (Ac2O3) can be obtained by heating the hydroxide at 500 °C or theoxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
at 1100 °C, in vacuum. Its crystal lattice is isotypic Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous ...
with the oxides of most trivalent rare-earth metals.
Halides
Actinium trifluoride can be produced either in solution or in solid reaction. The former reaction is carried out at room temperature, by adding hydrofluoric acid to a solution containing actinium ions. In the latter method, actinium metal is treated with hydrogen fluoride vapors at 700 °C in an all-platinum setup. Treating actinium trifluoride withammonium hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
at 900–1000 °C yields oxyfluoride AcOF. Whereas lanthanum oxyfluoride can be easily obtained by burning lanthanum trifluoride in air at 800 °C for an hour, similar treatment of actinium trifluoride yields no AcOF and only results in melting of the initial product.Meyer, pp. 87–88
:AcF3 + 2 NH3 + H2O → AcOF + 2 NH4F
Actinium trichloride is obtained by reacting actinium hydroxide or oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
with carbon tetrachloride vapors at temperatures above 960 °C. Similar to oxyfluoride, actinium oxychloride can be prepared by hydrolyzing actinium trichloride with ammonium hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
at 1000 °C. However, in contrast to the oxyfluoride, the oxychloride could well be synthesized by igniting a solution of actinium trichloride in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
.
Reaction of aluminium bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as al ...
and actinium oxide yields actinium tribromide:
:Ac2O3 + 2 AlBr3 → 2 AcBr3 + Al2O3
and treating it with ammonium hydroxide at 500 °C results in the oxybromide AcOBr.
Other compounds
Actinium hydride was obtained by reduction of actinium trichloride with potassium at 300 °C, and its structure was deduced by analogy with the corresponding LaH2 hydride. The source of hydrogen in the reaction was uncertain. Mixing monosodium phosphate (NaH2PO4) with a solution of actinium in hydrochloric acid yields white-colored actinium phosphate hemihydrate (AcPO4·0.5H2O), and heating actinium oxalate with hydrogen sulfide vapors at 1400 °C for a few minutes results in a black actinium sulfide Ac2S3. It may possibly be produced by acting with a mixture of hydrogen sulfide andcarbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
on actinium oxide at 1000 °C.
Isotopes
Naturally occurring actinium is composed of two radioactiveisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
s; (from the radioactive family of ) and (a granddaughter of ). decays mainly as a beta emitter
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For exam ...
with a very small energy, but in 1.38% of cases it emits an alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pr ...
, so it can readily be identified through alpha spectrometry. Thirty-six radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s have been identified, the most stable being with a half-life of 21.772 years, with a half-life of 10.0 days and with a half-life of 29.37 hours. All remaining radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
isotopes have half-lives that are less than 10 hours and the majority of them have half-lives shorter than one minute. The shortest-lived known isotope of actinium is (half-life of 69 nanoseconds) which decays through alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
. Actinium also has two known meta state
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
s. The most significant isotopes for chemistry are 225Ac, 227Ac, and 228Ac.
Purified comes into equilibrium with its decay products after about a half of year. It decays according to its 21.772-year half-life emitting mostly beta (98.62%) and some alpha particles (1.38%); the successive decay products are part of the actinium series
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly ...
. Owing to the low available amounts, low energy of its beta particles (maximum 44.8 keV) and low intensity of alpha radiation, is difficult to detect directly by its emission and it is therefore traced via its decay products.ActiniumGreat Soviet Encyclopedia
The ''Great Soviet Encyclopedia'' (GSE; ) is one of the largest Russian-language encyclopedias, published in the Soviet Union from 1926 to 1990. After 2002, the encyclopedia's data was partially included into the later ''Bolshaya rossiyskaya e ...
(in Russian) The isotopes of actinium range in atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
from 205 u () to 236 u ().
Occurrence and synthesis
 Actinium is found only in traces in
Actinium is found only in traces in uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
ores – one tonne of uranium in ore contains about 0.2 milligrams of 227Ac – and in thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
ores, which contain about 5 nanograms of 228Ac per one tonne of thorium. The actinium isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
227Ac is a transient member of the uranium-actinium series decay chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay dire ...
, which begins with the parent isotope 235U (or 239Pu) and ends with the stable lead isotope 207Pb. The isotope 228Ac is a transient member of the thorium series
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay direct ...
decay chain, which begins with the parent isotope 232Th and ends with the stable lead isotope 208Pb. Another actinium isotope (225Ac) is transiently present in the neptunium series
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly ...
decay chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay dire ...
, beginning with 237Np (or 233U) and ending with thallium ( 205Tl) and near-stable bismuth ( 209Bi); even though all primordial 237Np has decayed away, it is continuously produced by neutron knock-out reactions on natural 238U.
The low natural concentration, and the close similarity of physical and chemical properties to those of lanthanum and other lanthanides, which are always abundant in actinium-bearing ores, render separation of actinium from the ore impractical, and complete separation was never achieved. Instead, actinium is prepared, in milligram amounts, by the neutron irradiation of in a nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
.
:benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
solution from an aqueous solution of the radiation products, and the selectivity to a certain element is achieved by adjusting the pH (to about 6.0 for actinium). An alternative procedure is anion exchange with an appropriate resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on n ...
in nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, which can result in a separation factor of 1,000,000 for radium and actinium vs. thorium in a two-stage process. Actinium can then be separated from radium, with a ratio of about 100, using a low cross-linking cation exchange resin and nitric acid as eluant
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid chromatography experiment, for exam ...
.
225Ac was first produced artificially at the Institute for Transuranium Elements
The Institute for Transuranium Elements (ITU) is a nuclear research institute in Karlsruhe, Germany. The ITU is one of the seven institutes of the Joint Research Centre, a Directorate-General of the European Commission. The ITU has about 300 staf ...
(ITU) in Germany using a cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Jan ...
and at St George Hospital in Sydney using a linac
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of oscillating electric potentials along a linear be ...
in 2000. This rare isotope has potential applications in radiation therapy and is most efficiently produced by bombarding a radium-226 target with 20–30 MeV deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
ions. This reaction also yields 226Ac which however decays with a half-life of 29 hours and thus does not contaminate 225Ac.
Actinium metal has been prepared by the reduction of actinium fluoride with lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
vapor in vacuum at a temperature between 1100 and 1300 °C. Higher temperatures resulted in evaporation of the product and lower ones lead to an incomplete transformation. Lithium was chosen among other alkali metals because its fluoride is most volatile.Hammond, C. R. ''The Elements'' in
Applications
Owing to its scarcity, high price and radioactivity, 227Ac currently has no significant industrial use, but 225Ac is currently being studied for use in cancer treatments such as targeted alpha therapies. 227Ac is highly radioactive and was therefore studied for use as an active element of radioisotope thermoelectric generators, for example in spacecraft. The oxide of 227Ac pressed with beryllium is also an efficientneutron source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear p ...
with the activity exceeding that of the standard americium-beryllium and radium-beryllium pairs.Russell, Alan M. and Lee, Kok Loong (2005''Structure-property relations in nonferrous metals''
Wiley. , pp. 470–471 In all those applications, 227Ac (a beta source) is merely a progenitor which generates alpha-emitting isotopes upon its decay. Beryllium captures alpha particles and emits neutrons owing to its large cross-section for the (α,n) nuclear reaction: :
 225Ac is applied in medicine to produce in a reusable generator or can be used alone as an agent for
225Ac is applied in medicine to produce in a reusable generator or can be used alone as an agent for radiation therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radi ...
, in particular targeted alpha therapy (TAT). This isotope has a half-life of 10 days, making it much more suitable for radiation therapy than 213Bi (half-life 46 minutes). Additionally, 225Ac decays to nontoxic 209Bi rather than stable but toxic lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
, which is the final product in the decay chains of several other candidate isotopes, namely 227Th, 228Th, and 230U. Not only 225Ac itself, but also its daughters, emit alpha particles which kill cancer cells in the body. The major difficulty with application of 225Ac was that intravenous injection of simple actinium complexes resulted in their accumulation in the bones and liver for a period of tens of years. As a result, after the cancer cells were quickly killed by alpha particles from 225Ac, the radiation from the actinium and its daughters might induce new mutations. To solve this problem, 225Ac was bound to a chelating
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
agent, such as citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the ...
, ethylenediaminetetraacetic acid
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
(EDTA) or diethylene triamine pentaacetic acid
Pentetic acid or diethylenetriaminepentaacetic acid (DTPA) is an aminopolycarboxylic acid consisting of a diethylenetriamine backbone with five carboxymethyl groups. The molecule can be viewed as an expanded version of EDTA and is used similarly ...
(DTPA). This reduced actinium accumulation in the bones, but the excretion from the body remained slow. Much better results were obtained with such chelating agents as HEHA () or DOTA
''Dota'' is a series of strategy video games by Valve. The series began in 2003 with the release of ''Defense of the Ancients'' (''DotA''), a fan-developed multiplayer online battle arena (MOBA) mod for the video game '' Warcraft III: Reign o ...
() coupled to trastuzumab, a monoclonal antibody
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Monoclonal antibodies ...
that interferes with the HER2/neu
Receptor tyrosine-protein kinase erbB-2 is a protein that in humans is encoded by the ''ERBB2'' gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The human protein is also frequently refer ...
receptor. The latter delivery combination was tested on mice and proved to be effective against leukemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ...
, lymphoma, breast
The breast is one of two prominences located on the upper ventral region of a primate's torso. Both females and males develop breasts from the same embryological tissues.
In females, it serves as the mammary gland, which produces and sec ...
, ovarian
The ovary is an organ in the female reproductive system that produces an ovum. When released, this travels down the fallopian tube into the uterus, where it may become fertilized by a sperm. There is an ovary () found on each side of the body ...
, neuroblastoma and prostate cancers.
The medium half-life of 227Ac (21.77 years) makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters. The associated processes cannot be studied with the required accuracy by direct measurements of current velocities (of the order 50 meters per year). However, evaluation of the concentration depth-profiles for different isotopes allows estimating the mixing rates. The physics behind this method is as follows: oceanic waters contain homogeneously dispersed 235U. Its decay product, 231Pa, gradually precipitates to the bottom, so that its concentration first increases with depth and then stays nearly constant. 231Pa decays to 227Ac; however, the concentration of the latter isotope does not follow the 231Pa depth profile, but instead increases toward the sea bottom. This occurs because of the mixing processes which raise some additional 227Ac from the sea bottom. Thus analysis of both 231Pa and 227Ac depth profiles allows researchers to model the mixing behavior.
There are theoretical predictions that AcHx hydrides (in this case with very high pressure) are a candidate for a near room-temperature superconductor
A room-temperature superconductor is a material that is capable of exhibiting superconductivity at operating temperatures above , that is, temperatures that can be reached and easily maintained in an everyday environment. , the material with the h ...
as they have Tc significantly higher than H3S, possibly near 250 K.
Precautions
227Ac is highly radioactive and experiments with it are carried out in a specially designed laboratory equipped with a tightglove box
A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hand ...
. When actinium trichloride is administered intravenously to rats, about 33% of actinium is deposited into the bones and 50% into the liver. Its toxicity is comparable to, but slightly lower than that of americium and plutonium. For trace quantities, fume hoods with good aeration suffice; for gram amounts, hot cells with shielding from the intense gamma radiation emitted by 227Ac are necessary.
See also
*Actinium series
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly ...
Notes
References
Bibliography
* Meyer, Gerd and Morss, Lester R. (1991''Synthesis of lanthanide and actinide compounds''
Springer.
External links
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
NLM Hazardous Substances Databank – Actinium, Radioactive
Actinium
in {{Good article Chemical elements Chemical elements with face-centered cubic structure Actinides