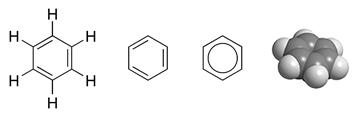
|
Phosphorus Pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride. Structure The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (''D''3h) symmetry. The hypervalent nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbitals (molecular orbital theory) or resonance ( valence bond theory). This trigonal bipyramidal structure persists in nonpolar solvents, such as CS2 and CCl4. In the solid state PCl5 is an ionic compound, formulated . In solutions of polar solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent. It has an "ether-like" odor, but commercial samples are typically contaminated with foul-smelling impurities.. It is of comparable toxicity to carbon monoxide. History In 1796, the German chemist Wilhelm August Lampadius (1772–1842) first prepared carbon disulfide by heating pyrite with moist charcoal. He called it "liquid sulfur" (''flüssig Schwefel''). The composition of carbon disulfide was finally determined in 1813 by the team of the Swedish chemist Jöns Jacob Berzelius (1779–1848) and the Swiss-British chemist Alexander Marcet (1770–1822). Their analysis was consistent with an empirical formula of CS2. Occurrence, manufacture, properties Small amounts of carbon disulfide are released by volcanic eru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccosine, cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as Group 14 hydride, its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have Tetrahedron#Isometries of irregular tetrahedra, lower symmetry. Tetrahedral molecules can be Chirality (chemistry), chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two Vector (mathematics and physics), vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira ( ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/MEDLINE/PubMed, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann End ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium () and carbonate () ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Ionic compounds usually form crystalline structures when solid. Ionic compounds containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases. Ionic compounds without these ions are also known as salts and can be formed by acid–base reactions. Ionic compounds can also be produced from their constituent ions by evaporation of thei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically incombustible at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Bond Theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. In contrast, molecular orbital theory has orbitals that cover the whole molecule. History Lothar Meyer in his 1864 book, ''Die modernen Theorien der Chemie'', contained an early version of the periodic table containing 28 elements, classified elements into six families by their valence—for the first time, elements had been grouped according to their valence. Works on organizing the elements by atomic weight, until then had been stymied by the widespread use of equivalent weights for the elements, rather than atomic weights. In 1916, G. N. Lewis proposed that a chemical bond forms by the interaction of two shared bonding electrons, with the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital Theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic nuclei in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons—the molecular orbitals—as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory (DFT) or Hartree–Fock (HF) models to the Schrödinger equation. Molecular orbital theory and valence bond theory are the foundation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an approximate solution to the Schrödi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |