|
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis-trans'' notation does not always correspond to ''E''–''Z'' isomerism, which is an ''absolute'' stereochemical description. In general, ''cis''–''trans'' stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor. Production Steam cracking The dominant technology for producing propylene is steam cracking. The same technology is applied to ethane to ethylene. These two conversions are the #2 and #1 processes in the chemical industry, as judged by their scale. In this process, propane undergoes dehydrogenation. The by-product is hydrogen: :CH3CH2CH3 → CH3CH=CH2 + H2 The yield of propene is about 85 m%. By-products are usually used as fuel for the propane dehydrogenation reaction. Steam cracking is one of the most energy-intensive industrial processes. The feedstock is naphtha or propane, especially in the Middle East, where there is an abundance of propane from oil/gas operations. Propene can be separated by fractional di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane () or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains are classified as small ( cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium ( cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formula for cycloalkanes is C''n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
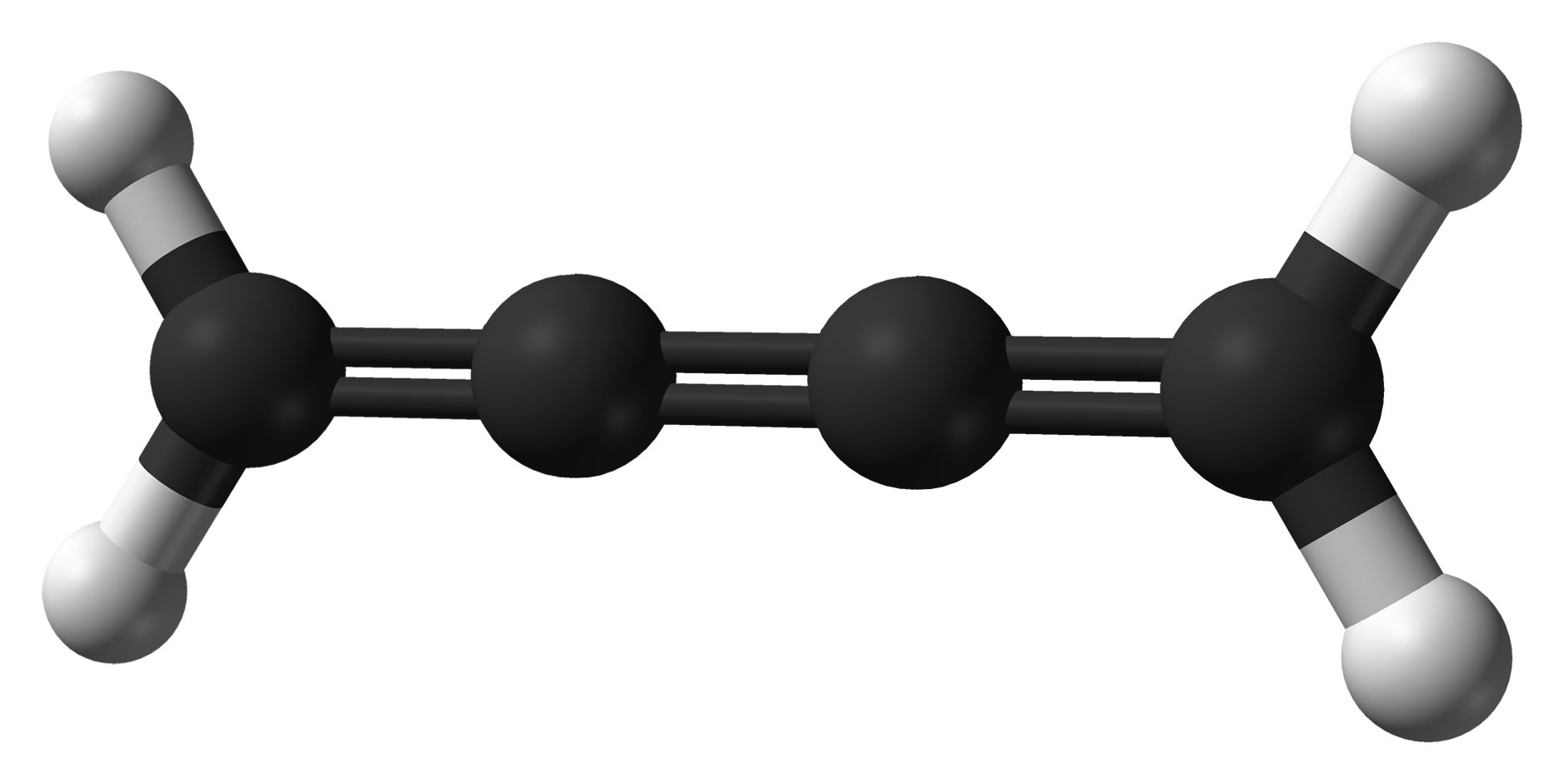
Cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular clouds and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalo vinylidene. Tetraphenylbutatriene was reported synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Butene
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting ''cis''/''trans''-isomerism (also known as (''E''/''Z'')-isomerism); that is, it exists as two geometric isomers ''cis''-but-2-ene ((''Z'')-but-2-ene) and ''trans-''but-2-ene ((''E'')-but-2-ene). It is a petrochemical, produced by the catalytic cracking of crude oil or the dimerization of ethylene. Its main uses are in the production of gasoline (petrol) and butadiene,. although some but-2-ene is also used to produce the solvent butanone via hydration to 2-butanol followed by oxidation. The two isomers are extremely difficult to separate by distillation because of the proximity of their boiling points (~4 °C for ''cis'' and ~1 °C for ''trans'' ). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open-chain Compound
In chemistry, an open-chain compound (also spelled as open chain compound) or acyclic compound (Greek prefix "α", ''without'' and "κύκλος", ''cycle'') is a compound with a linear structure, rather than a cyclic one. An open-chain compound having no side chains is called a straight-chain compound (also spelled as straight chain compound). Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic, with the latter often classified as aromatic. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix ''n-'' denotes the straight-chain isomer; for example, ''n''-butane is straight-chain butane, whereas ''i''-butane is isobutane. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring's closure involves a C-C bond. Having no rings (aromatic or otherwise), all open-chain compounds are aliphatic. Typically in bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyene
In organic chemistry, polyenes are poly- unsaturated, organic compounds that contain at least three alternating double () and single () carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, resulting in some unusual optical properties. Related to polyenes are dienes, where there are only two alternating double and single bonds. The following polyenes are used as antibiotics for humans: amphotericin B, nystatin, candicidin, pimaricin, methyl partricin, and trichomycin. Optical properties Some polyenes are brightly colored, an otherwise rare property for a hydrocarbon. Normally alkenes absorb in the ultraviolet region of a spectrum, but the absorption energy state of polyenes with numerous conjugated double bonds can be lowered such that they enter the visible region of the spectrum, resulting in compounds which are coloured (because they contain a chromophore). Thus many natural dyes contain linear polyenes. Chemical and el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |