|
X-RAY
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz ( to ) and energies in the range 145 eV to 124 keV. X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays. In many languages, X-radiation is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it on November 8, 1895. He named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . Spellings of ''X-ray(s)'' in English include the variants ''x-ray(s)'', ''xray(s)'', and ''X ray(s)''. The most familiar use of X-rays is checking for fractures (broken bones), but X-rays are also used in other ways. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crookes Tube
A Crookes tube (also Crookes–Hittorf tube) is an early experimental electrical discharge tube, with partial vacuum, invented by English physicist William Crookes and others around 1869-1875, in which cathode rays, streams of electrons, were discovered. Developed from the earlier Geissler tube, the Crookes tube consists of a partially evacuated glass bulb of various shapes, with two metal electrodes, the cathode and the anode, one at either end. When a high voltage is applied between the electrodes, cathode rays (electrons) are projected in straight lines from the cathode. It was used by Crookes, Johann Hittorf, Julius Plücker, Eugen Goldstein, Heinrich Hertz, Philipp Lenard, Kristian Birkeland and others to discover the properties of cathode rays, culminating in J.J. Thomson's 1897 identification of cathode rays as negatively charged particles, which were later named ''electrons''. Crookes tubes are now used only for demonstrating cathode rays. Wilhelm Röntgen discovered X ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic field, electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, Light, (visible) light, ultraviolet, X-rays, and gamma rays. All of these waves form part of the electromagnetic spectrum. Classical electromagnetism, Classically, electromagnetic radiation consists of electromagnetic waves, which are synchronized oscillations of electric field, electric and magnetic fields. Depending on the frequency of oscillation, different wavelengths of electromagnetic spectrum are produced. In a vacuum, electromagnetic waves travel at the speed of light, commonly denoted ''c''. In homogeneous, isotropic media, the oscillations of the two fields are perpendicular to each other and perpendicular to the direction of energy and wave propagation, forming a transverse wave. The position of an electromagnetic wave w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
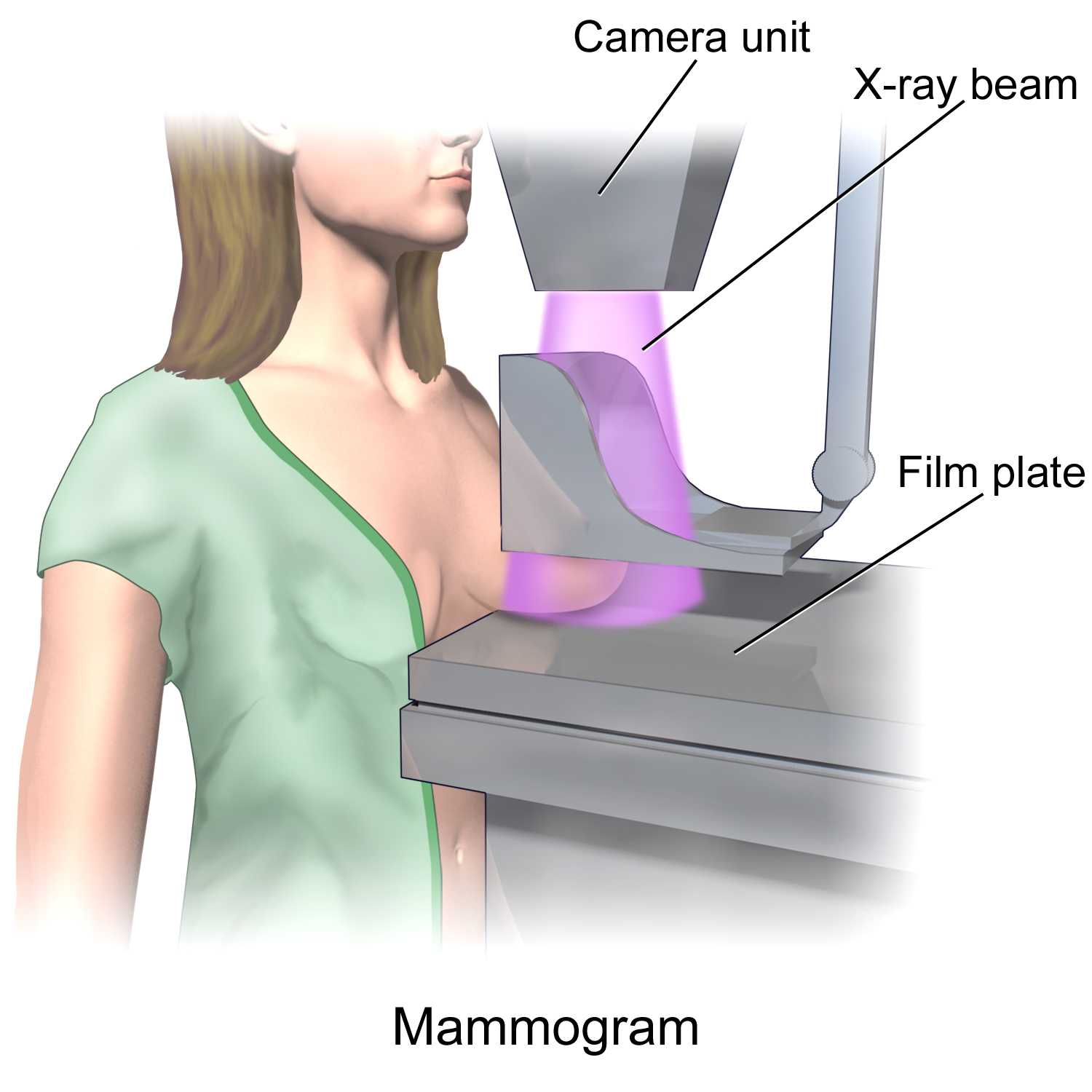
Mammography
Mammography (also called mastography) is the process of using low-energy X-rays (usually around 30 kVp) to examine the human breast for diagnosis and screening. The goal of mammography is the early detection of breast cancer, typically through detection of characteristic masses or microcalcifications. As with all X-rays, mammograms use doses of ionizing radiation to create images. These images are then analyzed for abnormal findings. It is usual to employ lower-energy X-rays, typically Mo (K-shell X-ray energies of 17.5 and 19.6 keV) and Rh (20.2 and 22.7 keV) than those used for radiography of bones. Mammography may be 2D or 3D (tomosynthesis), depending on the available equipment and/or purpose of the examination. Ultrasound, ductography, positron emission mammography (PEM), and magnetic resonance imaging (MRI) are adjuncts to mammography. Ultrasound is typically used for further evaluation of masses found on mammography or palpable masses that may or may not be seen on mammogr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelm Röntgen
Wilhelm Conrad Röntgen (; ; 27 March 184510 February 1923) was a German mechanical engineer and physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range known as X-rays or Röntgen rays, an achievement that earned him the inaugural Nobel Prize in Physics in 1901.Novelize, Robert. ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. 1997. p. 1. In honour of Röntgen's accomplishments, in 2004 the International Union of Pure and Applied Chemistry (IUPAC) named element 111, roentgenium, a radioactive element with multiple unstable isotopes, after him. The unit of measurement roentgen was also named after him. Biographical history Education He was born to Friedrich Conrad Röntgen, a German merchant and cloth manufacturer, and Charlotte Constanze Frowein. At age three his family moved to the Netherlands where his family lived. Röntgen attended high school at Utrecht Technical School in Utrecht, Netherland ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research. NASA was established in 1958, succeeding the National Advisory Committee for Aeronautics (NACA), to give the U.S. space development effort a distinctly civilian orientation, emphasizing peaceful applications in space science. NASA has since led most American space exploration, including Project Mercury, Project Gemini, the 1968-1972 Apollo Moon landing missions, the Skylab space station, and the Space Shuttle. NASA supports the International Space Station and oversees the development of the Orion spacecraft and the Space Launch System for the crewed lunar Artemis program, Commercial Crew spacecraft, and the planned Lunar Gateway space station. The agency is also responsible for the Launch Services Program, which provides oversight of launch operations and countdown management f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heinrich Hertz
Heinrich Rudolf Hertz ( ; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's Maxwell's equations, equations of electromagnetism. The unit of frequency, cycle per second, was named the "hertz" in his honor.IEC History . Iec.ch. Biography Heinrich Rudolf Hertz was born in 1857 in Hamburg, then a sovereign state of the German Confederation, into a prosperous and cultured Hanseatic (class), Hanseatic family. His father was Gustav Ferdinand Hertz. His mother was Anna Elisabeth Pfefferkorn. While studying at the Gelehrtenschule des Johanneums in Hamburg, Hertz showed an aptitude for sciences as well as languages, learning Arabic. He studied sciences and engineering in th ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philipp Lenard
Philipp Eduard Anton von Lenard (; hu, Lénárd Fülöp Eduárd Antal; 7 June 1862 – 20 May 1947) was a Hungarian-born German physicist and the winner of the Nobel Prize for Physics in 1905 for his work on cathode rays and the discovery of many of their properties. One of his most important contributions was the experimental realization of the photoelectric effect. He discovered that the energy (speed) of the electrons ejected from a cathode depends only on the wavelength, and not the intensity, of the incident light. Lenard was a nationalist and anti-Semite; as an active proponent of the Nazi ideology, he supported Adolf Hitler in the 1920s and was an important role model for the "Deutsche Physik" movement during the Nazi period. Notably, he labeled Albert Einstein's contributions to science as "Jewish physics". Early life and work Philipp Lenard was born in Pressburg (''Pozsony'', today's Bratislava), on 7 June 1862 in the Kingdom of Hungary. The Lenard family had origina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy In physics, the kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acc ... gained by a single electron accelerating from rest through an Voltage, electric potential difference of one volt in vacuum. When used as a Units of energy, unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the Electric charge, charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in Particle accelerator#Electrostatic particle accelerators, electrostatic particle accel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Morgan (actuary)
William Morgan, FRS (26 May OS? 1750 – 4 May 1833) was a British physician, physicist and statistician, who is considered the father of modern actuarial science. He is also credited with being the first to record the "invisible light" produced when a current is passed through a partly evacuated glass tube: "the first x-ray tube". Life He was born in Bridgend, Glamorgan, son to physician William Morgan and Sarah (sister of Richard Price). William's brother was George Cadogan Morgan. At eighteen he received medical training at Guy's Hospital, London, working also as an apothecary to pay his way. He did not complete his training, but after one year returned to Bridgend to join his father's practice. He was not popular with his father's patients: they thought him inexperienced and they resented receiving treatment from someone with a deformity—Morgan suffered from a club foot. After his father's death he left medicine and in 1774, on the recommendation of his mother's bro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






.jpg)