|
Tryptophan Metabolism
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. It is essential in humans, meaning that the body cannot synthesize it and it must be obtained from the diet. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Function Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skeletal Formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A skeletal formula shows the skeletal structure or skeleton of a molecule, which is composed of the skeletal atoms that make up the molecule. It is represented in two dimensions, as on a piece of paper. It employs certain conventions to represent carbon and hydrogen atoms, which are the most common in organic chemistry. An early form of this representation was first developed by organic chemist August Kekulé, while the modern form is closely related to and influenced by the Lewis structure of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures or Lewis–Kekulé structures. Skeletal formulae have become ubiquitous in organic chemistry, partly because they are relatively quick and simple to draw, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required for the correct development of animals, plants and fungi. Due to the broad definition of a hormone (as a signaling molecule that exerts its effects far from its site of production), numerous kinds of molecules can be classified as hormones. Among the substances that can be considered hormones, are eicosanoids (e.g. prostaglandins and thromboxanes), steroids (e.g. oestrogen and brassinosteroid), amino acid derivatives (e.g. epinephrine and auxin), protein or peptides (e.g. insulin and CLE peptides), and gases (e.g. ethylene and nitric oxide). Hormones are used to communicate between organs and tissues. In vertebrates, hormones are responsible for regulating a variety of physiological processes and behavioral activities such as diges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
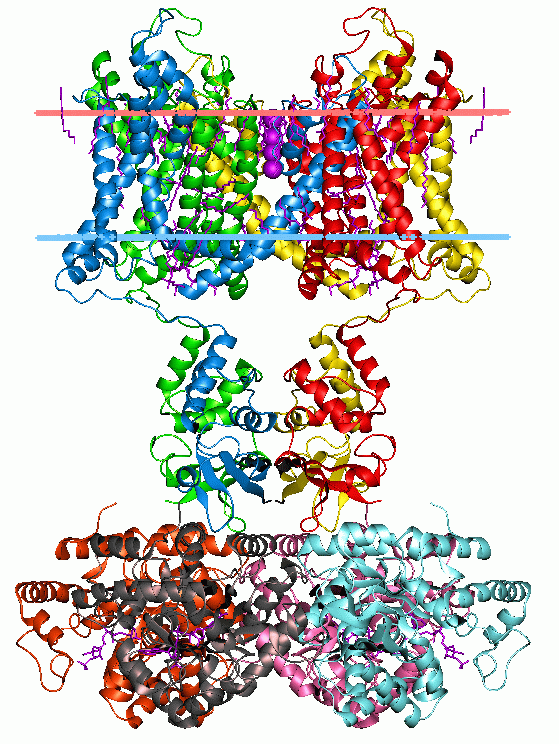
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane ( transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the targ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Biosynthesis
Protein biosynthesis (or protein synthesis) is a core biological process, occurring inside cells, balancing the loss of cellular proteins (via degradation or export) through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences. Protein synthesis can be divided broadly into two phases - transcription and translation. During transcription, a section of DNA encoding a protein, known as a gene, is converted into a template molecule called messenger RNA (mRNA). This conversion is carried out by enzymes, known as RNA polymerases, in the nucleus of the cell. In eukaryotes, this mRNA is initially produced in a premature form ( pre-mRNA) which undergoes post-transcriptional modifications to produce mature mRNA. The mature mRNA is exported from the cell nucleus via nuclear pores to the cyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan Metabolism
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. It is essential in humans, meaning that the body cannot synthesize it and it must be obtained from the diet. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Function Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Amino Acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine. Six other amino acids are considered conditionally essential in the human diet, meaning their synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline, and tyrosine. Six amino acids are non-essential (dispensable) in humans, meaning they can be synthesized in sufficient quantities in the body. These six are alanine, aspartic acid, asparagine, glutamic acid, serine, and selenocysteine (considered the 21st amino acid). Py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/PS_2A3_AA_Charges.html, accessed 12/2/2020 The species formed is the conjugate base of that acid. The complementary process, when a proton is added (transferred) to a Brønsted–Lowry base, is protonation (or hydronation). The species formed is the conjugate acid of that base. A species that can either accept or donate a proton is referred to as amphiprotic. An example is the H2O (water) molecule, which can gain a proton to form the hydronium ion, H3O+, or lose a proton, leaving the hydroxide ion, OH−. The relative ability of a molecule to give up a proton is measured by its p''K''a value. A low p''K''a value indicates that the compound is acidic and will easily give up its proton to a bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physiological PH
Physiology (; ) is the scientific study of functions and mechanisms in a living system. As a sub-discipline of biology, physiology focuses on how organisms, organ systems, individual organs, cells, and biomolecules carry out the chemical and physical functions in a living system. According to the classes of organisms, the field can be divided into medical physiology, animal physiology, plant physiology, cell physiology, and comparative physiology. Central to physiological functioning are biophysical and biochemical processes, homeostatic control mechanisms, and communication between cells. ''Physiological state'' is the condition of normal function. In contrast, ''pathological state'' refers to abnormal conditions, including human diseases. The Nobel Prize in Physiology or Medicine is awarded by the Royal Swedish Academy of Sciences for exceptional scientific achievements in physiology related to the field of medicine. Foundations Cells Although there are differences b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups. : With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion. Betaines are zwitterions that cannot isomerize to an all-neutral form, such as when the positive charge is located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot isomerize. Amino acids The equilibrium is established in two stages. In the first stage, a proton is transferred from the carboxyl group to a water molecule: :H2N(R)CO2H + H2O H2N(R)CO2- + H3O+ In the second stage, a proton is transferred from the hydronium ion to the amine group: :H2N(R)CO2- + H3O+ H3N+ (R)CO2- + H2O Overall, the reaction is an isomerization reaction :H2N(R)CO2H H3N+ (R)CO2- The ratio of the concentrations of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Codon
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links proteinogenic amino acids in an order specified by messenger RNA (mRNA), using transfer RNA (tRNA) molecules to carry amino acids and to read the mRNA three nucleotides at a time. The genetic code is highly similar among all organisms and can be expressed in a simple table with 64 entries. The codons specify which amino acid will be added next during protein biosynthesis. With some exceptions, a three-nucleotide codon in a nucleic acid sequence specifies a single amino acid. The vast majority of genes are encoded with a single scheme (see the RNA codon table). That scheme is often referred to as the canonical or standard genetic code, or simply ''the'' genetic code, though variant codes (such as in mitochondria) exist. History Ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |