|
Tegafur
Tegafur is a chemotherapeutic prodrug of 5-fluorouracil (5-FU) used in the treatment of cancers. It is a component of the combination drug tegafur/uracil. When metabolised, it becomes 5-FU. It was patented in 1967 and approved for medical use in 1972. Medical uses As a prodrug to 5-FU it is used in the treatment of the following cancers: * Stomach (when combined with gimeracil and oteracil) * Breast (with uracil) * Gallbladder * Lung (specifically adenocarcinoma, typically with uracil) * Colorectal (usually when combined with gimeracil and oteracil) * Head and neck * Liver (with uracil) * Pancreatic It is often given in combination with drugs that alter its bioavailability and toxicity such as gimeracil, oteracil or uracil. These agents achieve this by inhibiting the enzyme dihydropyrimidine dehydrogenase (uracil/gimeracil) or orotate phosphoribosyltransferase (oteracil). Adverse effects The major side effects of tegafur are similar to fluorouracil and include myelosuppre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tegafur/uracil
Tegafur/uracil is a chemotherapy drug combination used in the treatment of cancer, primarily bowel cancer. It is also called UFT or UFUR. Description UFT is a first generation dihydropyrimidine dehydrogenase (DPD) inhibitory fluoropyrimidine drug. It is an oral agent which combines uracil, a competitive inhibitor of DPD, with the 5-fluorouracil (5-FU) prodrug tegafur in a 4:1 molar ratio. Mechanism of action Excess uracil competes with 5-FU for DPD, thus inhibiting 5-FU catabolism. The tegafur is taken up by the cancer cells and breaks down into 5-FU, a substance that kills tumor cells. The uracil causes higher amounts of 5-FU to stay inside the cells and kill them. Tegafur is a type of antimetabolite. Uracil has also been stated to help protect the gastrointestinal tract from 5-FU toxicity and the related metabolites, with less side effects than 5-FU and other 5-FU related (pro)drugs. Tetrahydrofuran metabolites from the tegafur metabolism, unique among 5-FU based drugs, ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tegafur/uracil
Tegafur/uracil is a chemotherapy drug combination used in the treatment of cancer, primarily bowel cancer. It is also called UFT or UFUR. Description UFT is a first generation dihydropyrimidine dehydrogenase (DPD) inhibitory fluoropyrimidine drug. It is an oral agent which combines uracil, a competitive inhibitor of DPD, with the 5-fluorouracil (5-FU) prodrug tegafur in a 4:1 molar ratio. Mechanism of action Excess uracil competes with 5-FU for DPD, thus inhibiting 5-FU catabolism. The tegafur is taken up by the cancer cells and breaks down into 5-FU, a substance that kills tumor cells. The uracil causes higher amounts of 5-FU to stay inside the cells and kill them. Tegafur is a type of antimetabolite. Uracil has also been stated to help protect the gastrointestinal tract from 5-FU toxicity and the related metabolites, with less side effects than 5-FU and other 5-FU related (pro)drugs. Tetrahydrofuran metabolites from the tegafur metabolism, unique among 5-FU based drugs, ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tegafur/gimeracil/oteracil
Tegafur/gimeracil/oteracil, sold under the brand names Teysuno and TS-1, is a fixed-dose combination medication used for the treatment of advanced gastric cancer when used in combination with cisplatin, and also for the treatment of head and neck cancer, colorectal cancer, non–small-cell lung, breast, pancreatic, and biliary tract cancers. The most common severe side effects when used in combination with cisplatin include neutropenia (low levels of neutrophils, a type of white blood cell), anaemia (low red blood cell counts) and fatigue (tiredness). Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. Tegafur/gimeracil/oteracil (Teysuno) was approved for medical use in the European Union in March 2011. It has not been approved by the U.S. Food and Drug Administration (FDA). Medical uses In the European Union tegafur/gimeracil/oteracil is indicated in adults for the treatment of advanced gastr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capecitabine
Capecitabine, sold under the brand name Xeloda among others, is a chemotherapy medication used to treat breast cancer, gastric cancer and colorectal cancer. For breast cancer it is often used together with docetaxel. It is taken by mouth. Common side effects include abdominal pain, vomiting, diarrhea, weakness, and rashes. Other severe side effects include blood clotting problems, allergic reactions, heart problems such as cardiomyopathy, and low blood cell counts. It is not recommended in people with kidney problems. Use during pregnancy may result in harm to the fetus. Capecitabine, inside the body, is converted to 5-fluorouracil (5-FU) through which it acts. It belongs to the class of medications known as fluoropyrimidines, which also includes 5-FU and tegafur. Capecitabine was patented in 1992 and approved for medical use in 1998. It is on the World Health Organization's List of Essential Medicines. Medical uses It is used in the treatment of the following cancers: * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-fluorouracil
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts. Side effects of use by injection are common. They may include inflammation of the mouth, loss of appetite, low blood cell counts, hair loss, and inflammation of the skin. When used as a cream, irritation at the site of application usually occurs. Use of either form in pregnancy may harm the baby. Fluorouracil is in the antimetabolite and pyrimidine analog families of medications. How it works is not entirely clear, but it is believed to involve blocking the action of thymidylate synthase and thus stopping the production of DNA. Fluorouracil was patented in 1956 and came into medical use in 1962. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydropyrimidine Dehydrogenase
In enzymology, a dihydropyrimidine dehydrogenase (NADP+) () is an enzyme that catalyzes the chemical reaction :5,6-dihydrouracil + NADP+ \rightleftharpoons uracil + NADPH + H+ Thus, the two substrates of this enzyme are 5,6-dihydrouracil and NADP+, whereas its 3 products are uracil, NADPH, and H+. In humans the enzyme is encoded by the ''DPYD'' gene. It is the initial and rate-limiting step in pyrimidine catabolism. It catalyzes the reduction of uracil and thymine. It is also involved in the degradation of the chemotherapeutic drugs 5-fluorouracil and tegafur. It also participates in beta-alanine metabolism and pantothenate and coa biosynthesis. Terminology The systematic name of this enzyme class is 5,6-dihydrouracil:NADP+ 5-oxidoreductase. Other names in common use include: * dihydrothymine dehydrogenase *dihydrouracil dehydrogenase (NADP+) *4,5-dihydrothymine: oxidoreductase *DPD *DHPDH *dehydrogenase, dihydrouracil (nicotinamide adenine dinucleotide, phosphate) *DH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemotherapeutic
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs ( chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent (which almost always involves combinations of drugs) or it may aim to prolong life or to reduce symptoms ( palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called ''medical oncology''. The term ''chemotherapy'' has come to connote non-specific usage of intracellular poisons to inhibit mitosis (cell division) or induce DNA damage, which is why inhibition of DNA repair can augment chemotherapy. The connotation of the word chemotherapy excludes more selective agents that block extracellular signals ( signal transduction). The development of therapies with specific molecular or genetic targets ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotoxicity
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifically, a neurotoxin or neurotoxicant– alters the normal activity of the nervous system in such a way as to cause permanent or reversible damage to nervous tissue. This can eventually disrupt or even kill neurons, which are cells that transmit and process signals in the brain and other parts of the nervous system. Neurotoxicity can result from organ transplants, radiation treatment, certain drug therapies, recreational drug use, and exposure to heavy metals, bites from certain species of venomous snakes, pesticides, certain industrial cleaning solvents, fuels and certain naturally occurring substances. Symptoms may appear immediately after exposure or be delayed. They may include limb weakness or numbness, loss of memory, vision, and/or in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
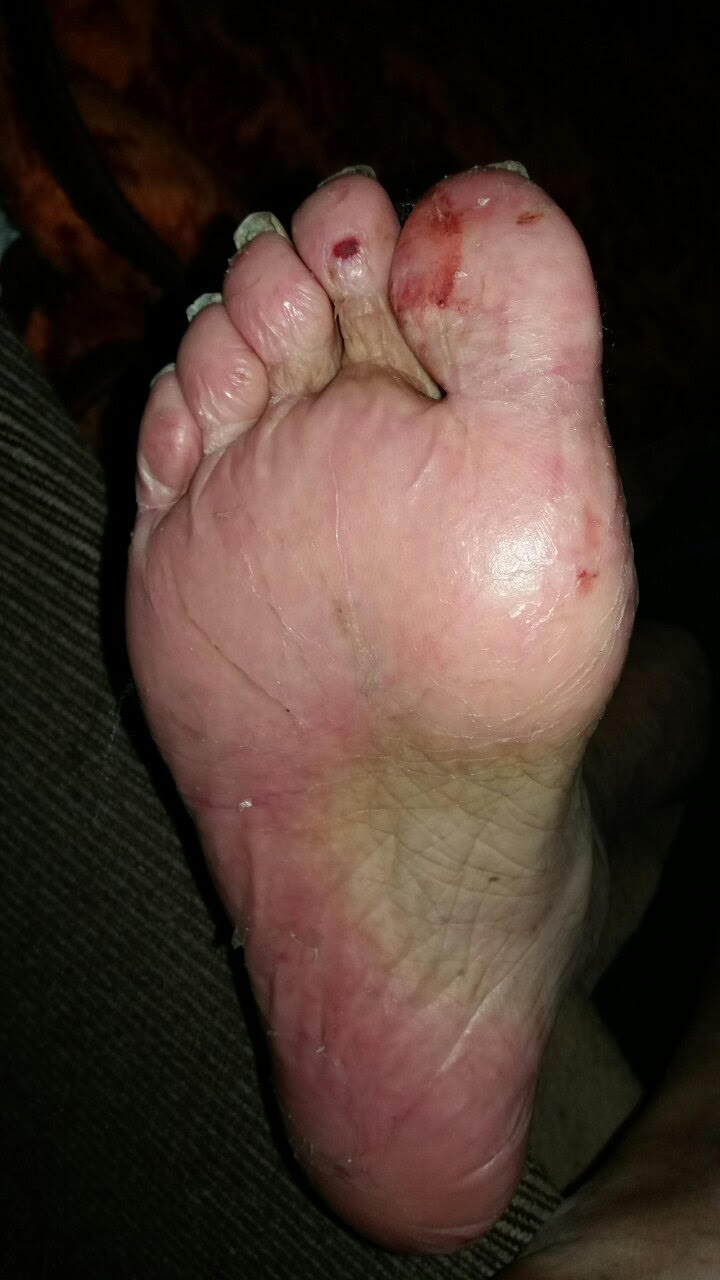
Hand-foot Syndrome
Chemotherapy-induced acral erythema, also known as palmar-plantar erythrodysesthesia or hand-foot syndrome is reddening, swelling, numbness and desquamation (skin sloughing or peeling) on palms of the hands and soles of the feet (and, occasionally, on the knees, elbows, and elsewhere) that can occur after chemotherapy in patients with cancer. Hand-foot syndrome is also rarely seen in sickle-cell disease. These skin changes usually are well demarcated. Acral erythema typically disappears within a few weeks after discontinuation of the offending drug.James, William; Berger, Timothy; Elston, Dirk (2005). ''Andrews' Diseases of the Skin: Clinical Dermatology''. (10th ed.). Saunders. . Signs and symptoms The symptoms can occur anywhere between days to months after administration of the offending medication, depending on the dose and speed of administration. The patient first experiences tingling and/or numbness of the palms and soles. This is followed 2-4 days later by bright redness ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymidylate Synthase
Thymidylate synthase (TS) () is an enzyme that catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). Thymidine is one of the nucleotides in DNA. With inhibition of TS, an imbalance of deoxynucleotides and increased levels of dUMP arise. Both cause DNA damage. Function The following reaction is catalyzed by thymidylate synthase: : 5,10-methylenetetrahydrofolate + dUMP \rightleftharpoons dihydrofolate + dTMP By means of reductive methylation, deoxyuridine monophosphate (dUMP) and N5,N10-methylene tetrahydrofolate are together used to form dTMP, yielding dihydrofolate as a secondary product. This provides the sole de novo pathway for production of dTMP and is the only enzyme in folate metabolism in which the 5,10-methylenetetrahydrofolate is oxidised during one-carbon transfer. The enzyme is essential for regulating the balanced supply of the 4 DNA precursors in normal DNA replication: defects in the enzyme activity affect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch, Peer ''Modern fluoroor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2A6
Cytochrome P450 2A6 (abbreviated CYP2A6) is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. CYP2A6 is the primary enzyme responsible for the oxidation of nicotine and cotinine. It is also involved in the metabolism of several pharmaceuticals, carcinogens, and a number of coumarin-type alkaloids. CYP2A6 is the only enzyme in the human body that appreciably catalyzes the 7-hydroxylation of coumarin, such that the formation of the product of this reaction, 7-hydroxycoumarin, is used as a probe for CYP2A6 activity. The CYP2A6 gene is part of a large cluster of cytochrome P450 genes from the CYP2A, CYP2B and CYP2F subfamilies on chromosome 19q. The gene was formerly referred to as CYP2A3; however, it has been renamed CYP2A6. Interactive pathway map Distribution CYP2A6 localizes to the endoplasmic reticulum and is found predominantly in the liver. Variability Significant interindividual variability in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |