|
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomers
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Isomerism
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals amou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
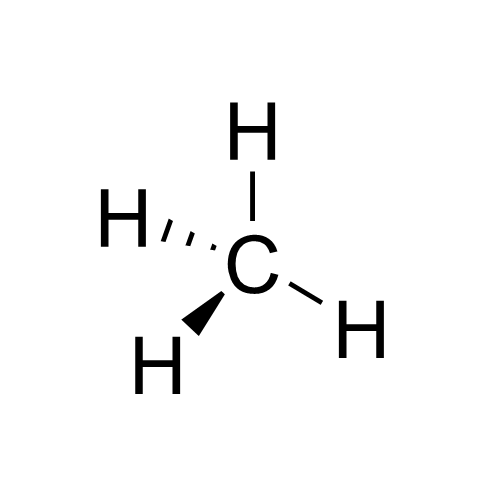
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoisomers
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer. Enantiomers Enantiomers, also known as optical isomers, are two stereoisomers that are related to each other by a reflection: they are mirror images of each other that are non-superposable. Human hands are a macroscopic analog of this. Every stereogenic center in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different optical is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereogenic
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups creates a new stereoisomer. Stereocenters are also referred to as stereogenic centers. A stereocenter is geometrically defined as a point (location) in a molecule; a stereocenter is usually but not always a specific atom, often carbon. Stereocenters can exist on chiral or achiral molecules; stereocenters can contain single bonds or double bonds. The number of hypothetical stereoisomers can be predicted by using 2''n'', with ''n'' being the number of tetrahedral stereocenters; however, exceptions such as meso compounds can reduce the prediction to below the expected 2''n''. Chirality centers are a type of stereocenter with four different substituent groups; chirality centers are a specific subset of stereocenters because they can only hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerism
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Stereochemistry
In chemistry, dynamic stereochemistry studies the effect of stereochemistry on the reaction rate of a chemical reaction. Stereochemistry is involved in: * stereospecific reactions * stereoselective or asymmetric reactions * racemisation In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. conta ... processes References * Carey, Francis A.; Sundberg, Richard J.; (1984). Advanced Organic Chemistry Part A Structure and Mechanisms (2nd ed.). New York N.Y.: Plenum Press {{ISBN, 0-306-41198-9. Stereochemistry Chemical kinetics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Carbon
An asymmetric carbon atom (chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms. Le Bel-van't Hoff rule states that the number of stereoisomers of an organic compound is 2n, where n represents the number of asymmetric carbon atoms (unless there is an internal plane of symmetry); a corollary of Le Bel and van't Hoff's simultaneously announced conclusions, in 1874, that the most probable orientation of the bonds of a carbon atom linked to four groups or atoms is toward the apexes of a tetrahedron, and that this accounted for all then-known phenomena of molecular asymmetry (which involved a carbon atom bearing four different atoms or groups). Knowing the number of asymmetric carbon atoms, one can calculate the maximum possible number of stereoisomers for any given molecule as follows: : If n is the number of asymmetric carbon atoms then the maximum number of isomers = 2n ( Le Bel-van't Hoff rule) As an example, malic acid has 4 car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joseph Le Bel
Joseph Achille Le Bel (21 January 1847 in Pechelbronn – 6 August 1930, in Paris, France) was a French chemist. He is best known for his work in stereochemistry. Le Bel was educated at the École Polytechnique in Paris. In 1874 he announced his theory outlining the relationship between molecular structure and optical activity. This discovery laid the foundation of the science of stereochemistry, which deals with the spatial arrangement of atoms in molecules. This hypothesis was put forward in the same year by the Dutch physical chemist Jacobus Henricus van 't Hoff and is currently known as Le Bel–van't Hoff rule. Le Bel wrote ''Cosmologie Rationelle'' (Rational Cosmology) in 1929. Works * * * See also *Hexamethylbenzene *Optical rotation Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain mate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartaric Acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid is an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid. History Tartaric acid has been known to winemakers for centuries. However, the chemical process for extraction was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele. Tartaric acid played an important role in the discovery of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |